[1] L. G. Bach, M. L. N. Thi, N. T. Son, Q. B. Bui, H.-T. Nhac-Vu, and P. H. Ai-Le, “Mesoporous gold nanoparticles supported cobalt nanorods as a free-standing electrochemical sensor for sensitive hydrogen peroxide detection,” J. Electroanal. Chem., vol. 848, 2019. https://doi.org/10.1016/j.jelechem.2019.113359
[2] E. S. Abdel-Halim and S.S. Al-Deyab, “One-step bleaching process for cotton fabrics using activated hydrogen peroxide,” Carbohydr. Polym., vol. 92, pp. 1844–1849, 2013. https://doi.org/10.1016/j.carbpol.2012.11.045
[3] S. N. L. Lima, I. S. Ribeiro, M. A. Grisotto, E. S. Fernandes, V. Hass, R. R. J. Tavarez, S. C. S. Pinto, D. M. Lima, A. D. Loguercio and M. C. Bandeca, “Evaluation of several clinical parameters after bleaching with hydrogen peroxide at different concentrations: A randomized clinical trial,” J. Dent., vol. 68, pp. 91–97, 2018. https://doi.org/10.1016/j.jdent.2017.11.008
[4] Z. Li, H. Dou, Y. Fu and M. Qin, “Improving the hydrogen peroxide bleaching efficiency of aspen chemithermomechanical pulp by using chitosan,” Carbohydr. Polym., vol. 132, pp. 430–436, 2015. https://doi.org/10.1016/j.carbpol.2015.06.062
[5] F. Liu, L. Yang, X. Yin, X. Liu, L. Ge and F. Li, “A facile homogeneous electrochemical biosensing strategy based on displacement reaction for intracellular and extracellular hydrogen peroxide detection,” Biosens. Bioelectron., vol. 141, 2019. https://doi.org/10.1016/j.bios.2019.111446
[6] L. K. Ndungu, J. H. Steele, T. L. Hancock, R. D. Bartleson, E. C. Milbrandt, M. L. Parsons and H. Urakawa, “Hydrogen peroxide measurements in subtropical aquatic systems and their implications for cyanobacterial blooms,” Ecol. Eng., vol. 138, pp. 444–453, 2019. https://doi.org/10.1016/j.ecoleng.2019.07.011
[7] C. Peña-Bautista, M. Vento, M. Baquero and C. Cháfer-Pericás, “Lipid peroxidation in neurodegeneration,” Clin. Chim. Acta., vol. 497, pp. 178–188, 2019. https://doi.org/10.1016/j.cca.2019.07.037
[8] Y. Wen, F. Huo and C. Yin, “Organelle targetable fluorescent probes for hydrogen peroxide,” Chinese Chem. Lett., vol. 30, no. 10, 2019. https://doi.org/10.1021/ja100117u
[9] L. Zhang, P. Zhao, C. Yue, Z. Jin, Q. Liu, X. Du and Q. He, “Sustained release of bioactive hydrogen by Pd hydride nanoparticles overcomes Alzheimer’s disease,” Biomaterials, vol. 197, pp. 393–404, 2019. https://doi.org/10.1016/j.biomaterials.2019.01.037
[10] S. Farzana, V. Ganesh and S. Berchmans, “A Sensing Platform for Direct Electron Transfer Study of Horseradish Peroxidase,” J. Electrochem. Soc., vol. 160, no. 9, pp. H573–H580, 2013. https://iopscience.iop.org/article/10.1149/2.038309jes
[11] M. Moyo, J. O. Okonkwo and N. M. Agyei, “A novel hydrogen peroxide biosensor based on adsorption of horseradish peroxidase onto a nanobiomaterial composite modified glassy carbon electrode,” Electroanalysis, vol. 25, no. 8, pp. 1946–1954, 2013. https://doi.org/10.1002/elan.201300165
[12] G. S. Lai, H. L. Zhang and D. Y. Han, “Amperometric hydrogen peroxide biosensor based on the immobilization of horseradish peroxidase by carbon-coated iron nanoparticles in combination with chitosan and cross-linking of glutaraldehyde,” Microchim. Acta, vol. 165, pp. 159–165, 2009.
[13] W. Chen, S. Cai, Q. Q. Ren, W. Wen, and Y. Di. Zhao, “Recent advances in electrochemical sensing for hydrogen peroxide: A review,” Analyst, vol. 137, no. 1, pp. 49–58, 2012. https://doi.org/10.1039/C1AN15738H
[14] I. Y. Sakharov, “Palm tree peroxidases,” Biochem. Biokhimiia, vol. 69, pp. 823–829, 2004. https://doi.org/10.1023/b:biry.0000040213.91951.bc
[15] P. A. Uribe, C. C. Ortiz, D. A. Centeno, J. J. Castillo, S. I. Blanco, and J. A. Gutierrez, “Self-assembled Pt screen printed electrodes with a novel peroxidase Panicum maximum and zinc oxide nanoparticles for H.O. detection,” Colloids Surfaces A Physicochem. Eng. Asp., vol. 561, pp. 18–24, 2019. https://doi.org/10.1016/j.colsurfa.2018.10.051
[16] A. E. Orduz, J. A. Gutiérrez, S. I. Blanco, J. J. Castillo and J. C. Salcedo-Reyes, “Amperometric detection of triclosan with screen-printed carbon nanotube electrodes modified with Guinea Grass .Panicum maximum) peroxidase,” Universit. Scient., vol. 24, pp. 363–379, 2019. https://doi.org/10.11144/Javeriana.SC24-2.adot
[17] M. Li, S. Xu, M. Tang, L. Liu, F. Gao and Y. Wang, “Direct electrochemistry of horseradish peroxidase on graphene-modified electrode for electrocatalytic reduction towards H.O.,” Electrochim. Acta, vol. 56, no. 3, pp. 1144–1149, 2011. https://doi.org/10.1016/j.electacta.2010.10.034
[18] X. Bo, M. Zhou and L. Guo, “Electrochemical sensors and biosensors based on less aggregated graphene,” Biosens. Bioelectron, vol. 89, no. 1, pp. 167–186, 2017. https://doi.org/10.1016/j.bios.2016.05.002
[19] A. Bonanni, A. H. Loo and M. Pumera, “Graphene for impedimetric biosensing,” TrAC - Trends Anal. Chem., vol. 37, pp. 12–21, 2012. https://doi.org/10.1016/j.trac.2012.02.011
[20] B. Dinesh, V. Mani, R. Saraswathi and S.-M. Chen, “Direct electrochemistry of cytochrome c immobilized on a graphene oxide–carbon nanotube composite for picomolar detection of hydrogen peroxide,” RSC Adv., vol. 4, no. 54, 2014. https://doi.org/10.1039/C4RA02789B
[21] P. Olejnik, A. Świetlikowska, M. Gniadek and B. Palys, “Electrochemically Reduced Graphene Oxide on Electrochemically Roughened Gold as a Support for Horseradish Peroxidase,” J. Phys. Chem., vol. 118, pp. 129731-29738, 2014. https://doi.org/10.1021/jp507227z
[22] V. Vukojević, S. Djurdjić, M. Ognjanović, M. Fabian, A. Samphao, K. Kalcher and D.M. Stanković, “Enzymatic glucose biosensor based on manganese dioxide nanoparticles decorated on graphene nanoribbons,” J. Electroanal. Chem., vol. 823, pp. 610–616, 2018. https://doi.org/10.1016/j.jelechem.2018.07.013
[23] L. Li, H. Lu and L. Deng, “A sensitive NADH and ethanol biosensor based on graphene-Au nanorods nanocomposites,” Talanta, vol. 113, pp. 1–6, 2013. https://doi.org/10.1016/j.talanta.2013.03.074
[24] Y. Huang, Y. Xue. J. Zeng, S. Li, Z. Wang, C. Dong, G. Li, J. Liang, Z. Zhou. “Non-enzymatic electrochemical hydrogen peroxide biosensor based on reduction graphene oxide-persimmon tannin‑platinum nanocomposite”, Mater. Sci. Eng. C, vol. 92, pp. 590-598, 2018. https://doi.org/10.1016/j.msec.2018.07.021
[25] Z. Lu, L. Wu, X. Dai, Y. Wang, M. Sun, C. Zhou, H. Du and H. Rao. “Novel flexible bifunctional amperometric biosensor based on laser engraved porous graphene array electrodes: Highly sensitive electrochemical determination of hydrogen peroxide and glucose”. J. Hazard. Mater, vol. 402, pp. 123774, 2021. https://doi.org/10.1016/j.jhazmat.2020.123774
[26] S. Pandey, S. Sachan and S. Singh. “Electrochemically reduced graphene oxide modified with electrodeposited thionine and horseradish peroxidase for hydrogen peroxide sensing and inhibitive measurement of chromium”. Mat. Sci. Energy Techn, vol. 2, no. 3, pp. 676-686, 2019. https://doi.org/10.1016/j.mset.2019.08.001
[27] P. R. Matheus, J. M. Abad and V. M. Fernández, “Modification of gold surfaces for the oriented immobilization of recombinant form of horseradish peroxidase,” Rev. Téc. Ing. Univ. Zulia, vol. 30, no. 3, pp. 225–235, 2007. https://www.produccioncientificaluz.org/index.php/tecnica/article/view/6195
[28] D. A. Centeno, X. H. Solano and J. J. Castillo, “A new peroxidase from leaves of guinea grass (Panicum maximum): A potential biocatalyst to build amperometric biosensors,” Bioelectrochemistry, vol. 116, pp. 33-38, 2017.
[29] G. Battistuzzi, M. Bellei, C. A. Bortolotti and M. Sola, “Redox properties of heme peroxidases,” Arch. Biochem. Biophys., vol. 500, no. 1, pp. 21–36, 2010. https://doi.org/10.1016/j.abb.2010.03.002
[30] L. Lu, Y. Dong, J. Wang, Q. Li and, X. Wu, “Direct electrochemistry and bioelectrocatalysis of horseradish peroxidase entrapped in a self-supporting nanoporous gold electrode: A new strategy to improve the orientation of immobilized enzymes,” Anal. Methods., vol 7, no. 16, pp. 6686–6694, 2015. https://doi.org/10.1039/C5AY01333J
[31] Y.-X. Sun, J.-T. Zhang, S.-W. Huang and S.-F. Wang, “Hydrogen peroxide biosensor based on the bioelectrocatalysis of horseradish peroxidase incorporated in a new hydrogel film,” Sensors Actuators, B Chem., vol. 124, no. 2, pp. 494–500, 2007.
[32] Y. Wang, Z. Wang, Y. Rui and M. Li, “Horseradish peroxidase immobilization on carbon nanodots/CoFe layered double hydroxides: Direct electrochemistry and hydrogen peroxide sensing,” Biosens. Bioelectron., vol. 64, pp. 57–62, 2015. https://doi.org/10.1016/j.bios.2014.08.054
[33] Y. Xu, C. Hu and S. Hu, “A hydrogen peroxide biosensor based on direct electrochemistry of hemoglobin in Hb-Ag sol films,” Sensors Actuators, B. Chem., vol. 130, no. 2, pp. 816–822, 2008. https://doi.org/10.1016/j.snb.2007.10.048
[34] L. Shi, X. Liu, W. Niu, H. Li, S. Han, J. Chen and G. Xu, “Hydrogen peroxide biosensor based on direct electrochemistry of soybean peroxidase immobilized on single-walled carbon nanohorn modified electrode,” Biosens. Bioelectron., vol. 24, no. 5, pp. 1159–1163, 2009. https://doi.org/10.1016/j.bios.2008.07.001
[35] C. Ren, Y. Song, Z. Li and G. Zhu, “Hydrogen peroxide sensor based on horseradish peroxidase immobilized on a silver nanoparticles/cysteamine/gold electrode,” Anal. Bioanal. Chem., vol. 381, no. 6, pp. 1179–1185, 2005. https://doi.org/10.1007/s00216-004-3032-0
[36] F. Chekin, N. Leiva, J. B. Raoof, L. Gorton and L. Bülow, “Direct electrochemistry and bioelectrocatalysis of a class II non-symbiotic plant haemoglobin immobilised on screen-printed carbon electrodes,” Anal. Bioanal. Chem., vol. 398, no. 4, pp. 1643–1649, 2010. https://doi.org/10.1007/s00216-010-3800-y


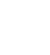
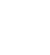



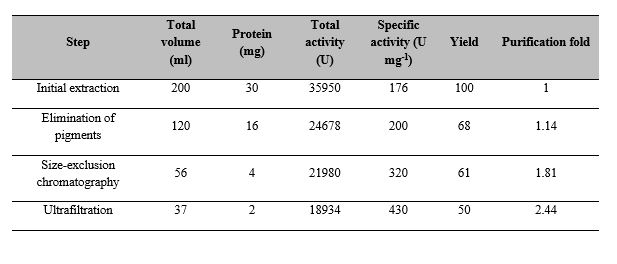
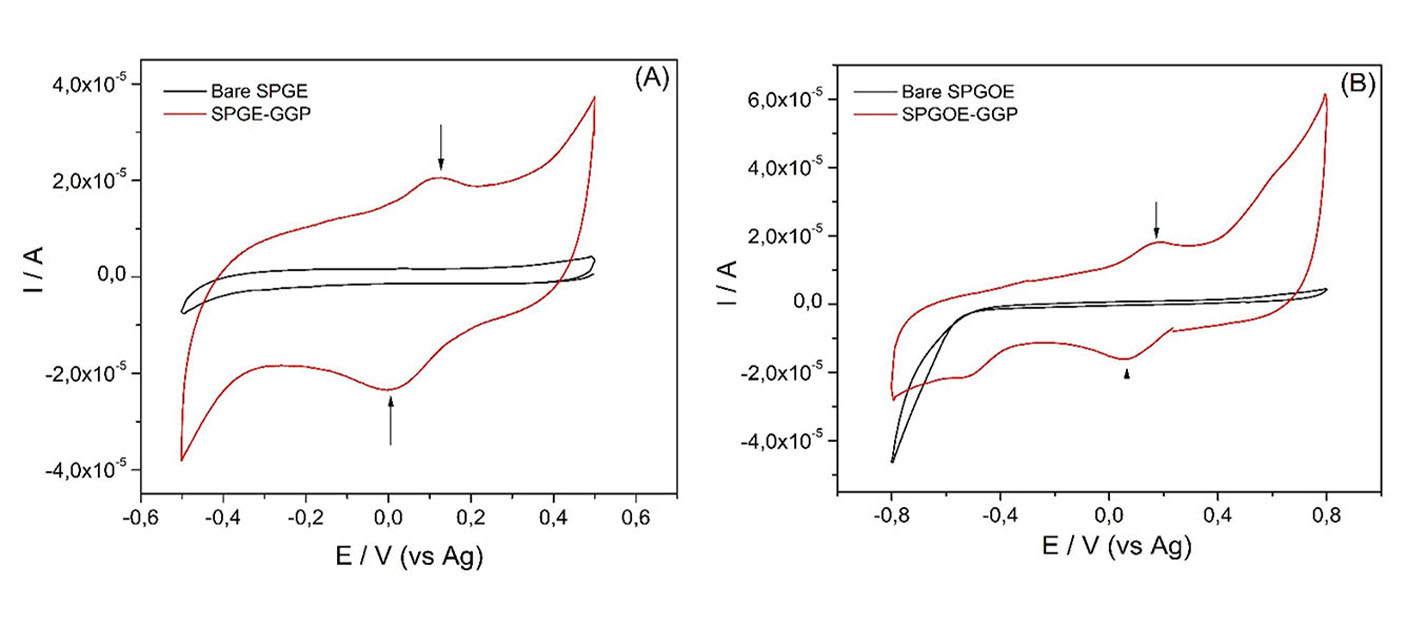
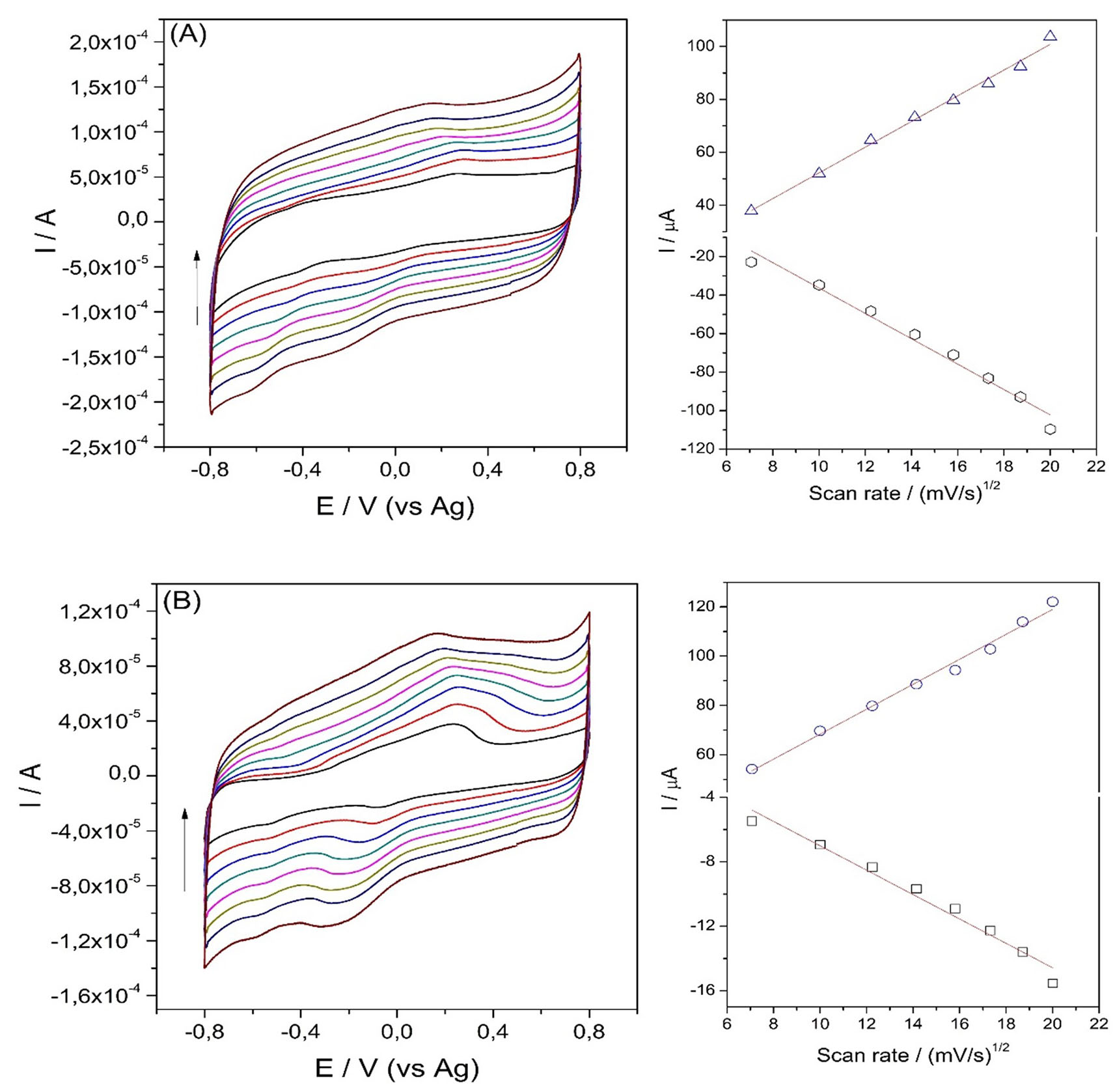
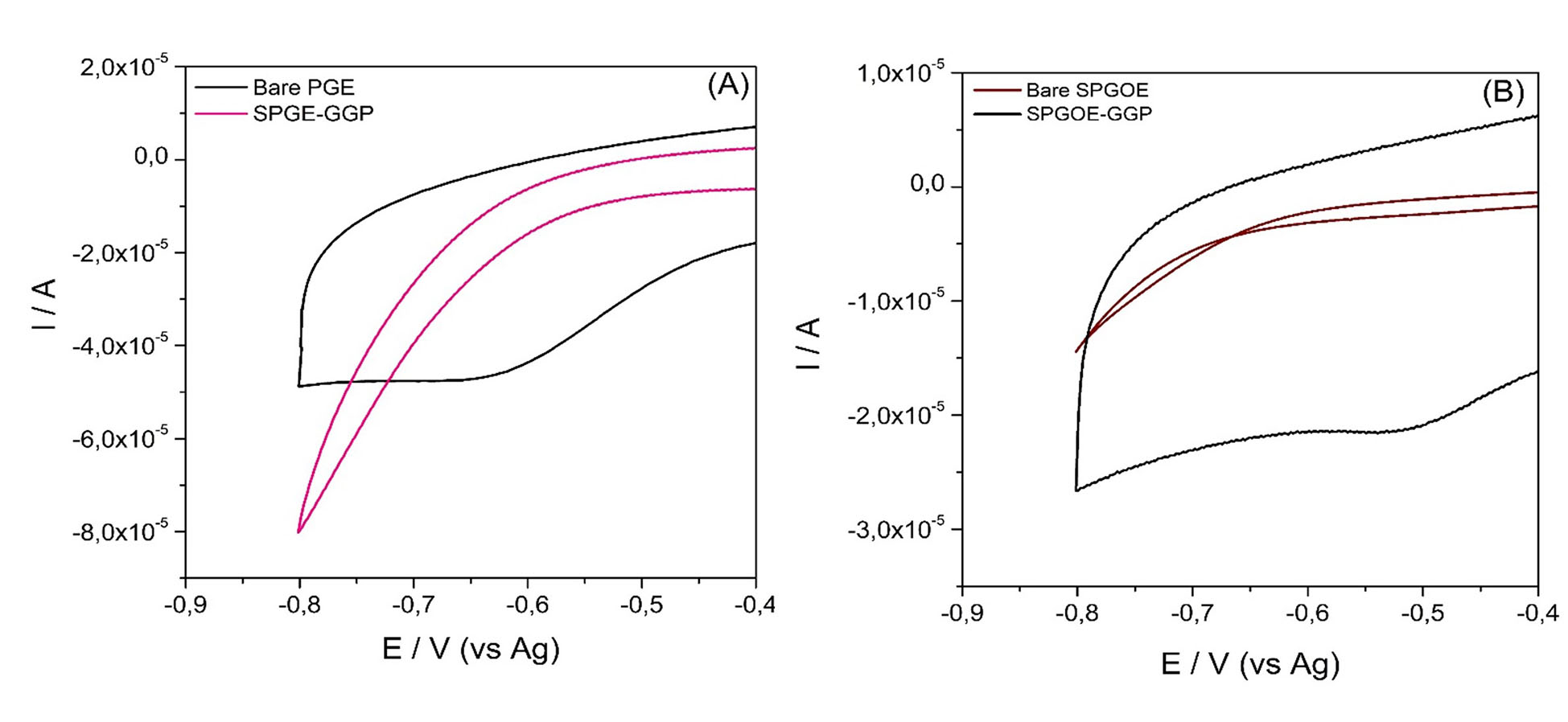
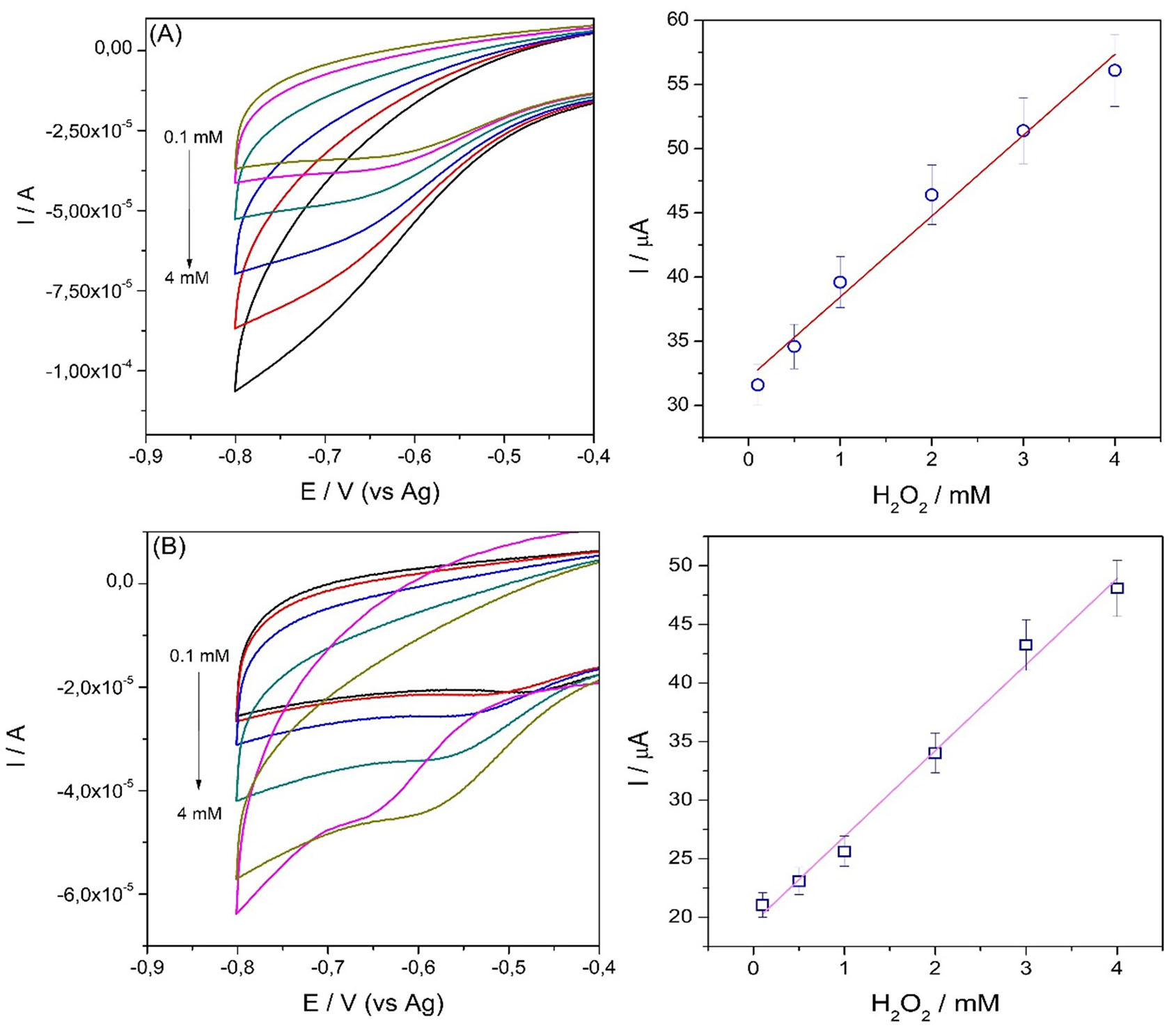
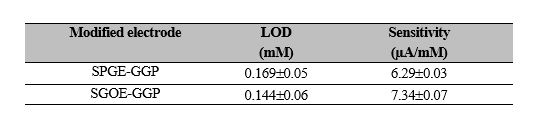
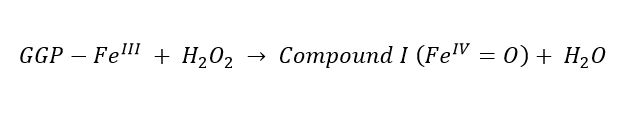 (1)
(1)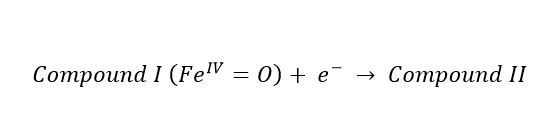 (2)
(2)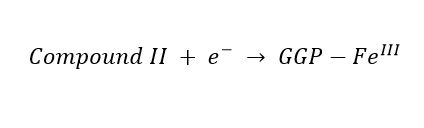 (3)
(3)