Introduction
Diabetic polyneuropathy (DPN) is the most common chronic microvascular complication of diabetes mellitus (DM) (1-3). According to data from the World Health Organization, from 1980 to 2014, 314 million new cases of DM were recorded worldwide, increasing from 108 million in 1980 to 422 million in 2014 (4,5). Of these, approximately 40% to 50% may develop DPN (6,7).
According to the American Diabetes Association, all patients diagnosed with type 1 DM with a disease duration of ≥5 years and all patients diagnosed with type 2 DM should be evaluated annually for DPN through anamnesis and simple clinical tests (8). To date, multiple tests have been developed to facilitate the clinical diagnosis of DPN, with some of the most widely used being the Michigan Neuropathy Screening Instrument (MNSI) (9,10) and the Semmes-Weinstein Monofilament Test (10 g) (8,9,11,12). Additionally, electrophysiological testing and evaluation by a clinical neurology specialist are recommended in cases of atypical presentation or diagnostic uncertainty (8,13,14).
Nerve conduction studies and electromyography can provide early information for the early detection of abnormalities in action potential amplitudes. Moreover, these tools offer a more precise classification of DPN, which can be motor, sensory, or mixed. Neurophysiological studies help differentiate between axonal and demyelinating neuropathies while also assisting in ruling out other neurological conditions that may present with similar symptoms (e.g., Guillain-Barré syndrome or chronic inflammatory demyelinating neuropathy) (15).
The MNSI test consists of two parts: the first includes a 15-question questionnaire aimed at identifying possible neuropathic symptoms, and the second involves a targeted physical examination. A clinical diagnosis of DPN can be established with seven or more affirmative responses on the questionnaire or a score of two or more on the physical examination (sensitivity: 65% and specificity: 83%). Meanwhile, the Semmes-Weinstein Monofilament Test (10 g) diagnoses the presence of DPN with sensitivity and specificity ranging from 75% to 98% (9-12,16).
The objective of the present study was to determine the proportion of DPN and its possible associated factors in a group of hospitalized patients at a high-complexity institution in Bogotá, Colombia.
Materials and Methods
A cross-sectional analytical observational study was conducted, including 132 patients diagnosed with type 2 DM who were hospitalized in a high-complexity institution in Bogotá, Colombia, during the first half of 2019. The sample size was calculated using individual proportion estimation through confidence intervals. Patients aged over 18 years with a diagnosis of type 2 DM who were hospitalized at the time of the study and voluntarily agreed to participate were included. Patients with a history of cerebrovascular disease, spinal cord or spinal nerve disease, dementia or any type of cognitive impairment, ongoing pregnancy, or bilateral transtibial amputation were excluded.
The diagnostic criteria for DPN, according to the MNSI test, were: seven or more affirmative responses on the questionnaire or two or more positive points on the physical examination. The cutoff point for DPN diagnosis using the monofilament test was four or more altered sensitivity points. To improve diagnostic specificity for DPN, both tests (MNSI and monofilament) were used in combination (8,11,17-19).
Data Collection Instrument
Data were collected using an instrument composed of four sections: the first included information on sociodemographic characteristics; the second, information on the patient's clinical history; the third, the MNSI and Semmes-Weinstein monofilament test (10 g); and the fourth, the ulceration-amputation risk scale from the Colombian Guide for the Prevention, Diagnosis, and Treatment of Diabetic Foot (13).
Statistical Analysis
The data analysis was conducted using the statistical software SPSS, version 25.0 (IBM Corp., Armonk, NY, USA). According to their distribution, quantitative variables were described using measures of central tendency (mean and median) and dispersion (standard deviation and interquartile range), while qualitative variables of interest were reported with absolute and relative frequency distributions. To compare the proportion of DPN observed in this study with that reported in the literature, the chi-square test (χ²) was used. Subsequently, the association between potential related factors and the outcome (DPN diagnosis) was analyzed using the chi-square test for independence and the estimation of association measures (OR). Finally, a multiple logistic regression model was adjusted to determine which factors were independently associated with DPN diagnosis.
Ethical Considerations
This study adhered to national and international bioethical principles, as established by Colombian law under Resolution 08430 of 1993. The research was reviewed, approved, and supervised by the Institutional Ethics Committee, which also provided the legal guidelines for the development of the informed consent process. Regarding the use of personal data, this study complied with the provisions of Colombian Law 1581 of 2012, which safeguards data privacy and security.
Results
A total of 132 patients diagnosed with type 2 DM were analyzed (67 men and 65 women). The mean age was 65.3 ± 13.7 years. The median time from DM diagnosis to inclusion in this study was 10 years (IQR: 6-15). Among the total population, 62.9% (n = 83) resided in urban areas, 53% (n = 70) had only completed primary education, and 49.2% (n = 65) were unemployed. Regarding comorbidities, 78% of patients (n = 103) had at least one condition associated with DM, with hypertension (HTN) being the most frequent (66.7%; n = 88), followed by chronic kidney disease (CKD) (32.6%; n = 43) and a history of plantar ulceration (18.9%; n = 25).
Regarding pharmacological management of DM, 88.3% of patients (n = 117) were receiving some form of hypoglycemic medication. The most commonly used pharmacological groups included biguanides (e.g., metformin) and insulin (e.g., ultra-rapid, intermediate, and long-acting insulin). In the total sample, 11.3% of patients (n = 15) did not exhibit adequate treatment adherence. Among them, 53.3% (n = 8) were receiving some form of hypoglycemic medication but could not recall which one, and 52.3% (n = 7) were not receiving any treatment. Regarding glycemic control, 72% of individuals (n = 95) had an HbA1c value ≥7% (Table 1).
Table 1.
Clinical and Demographic
Characteristics
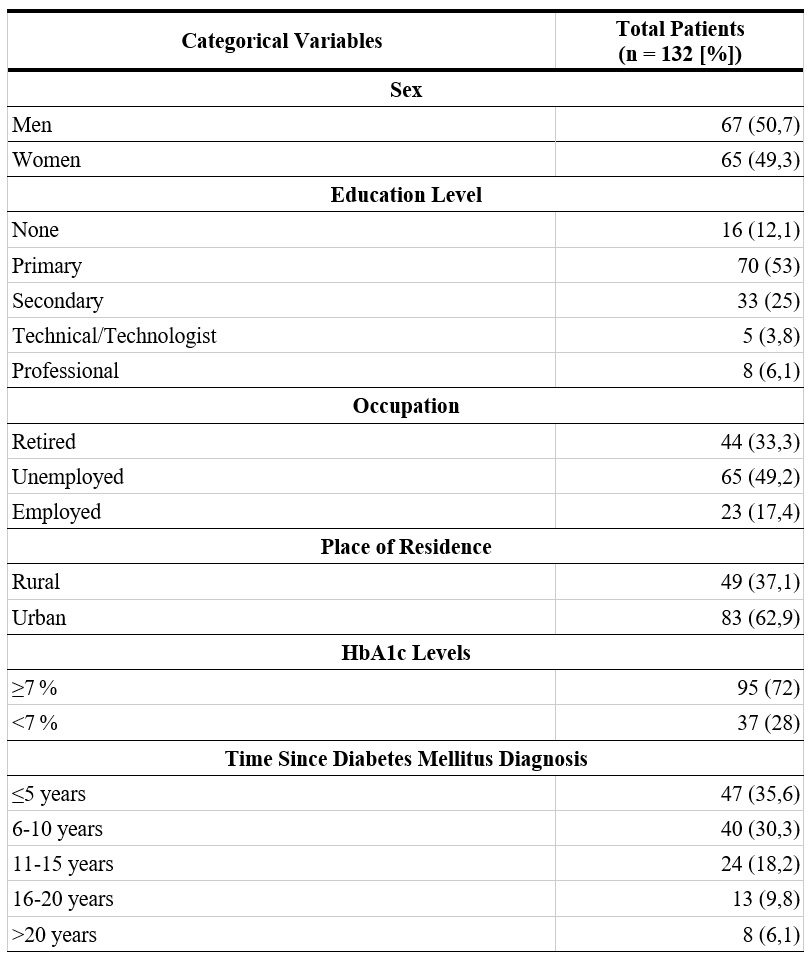
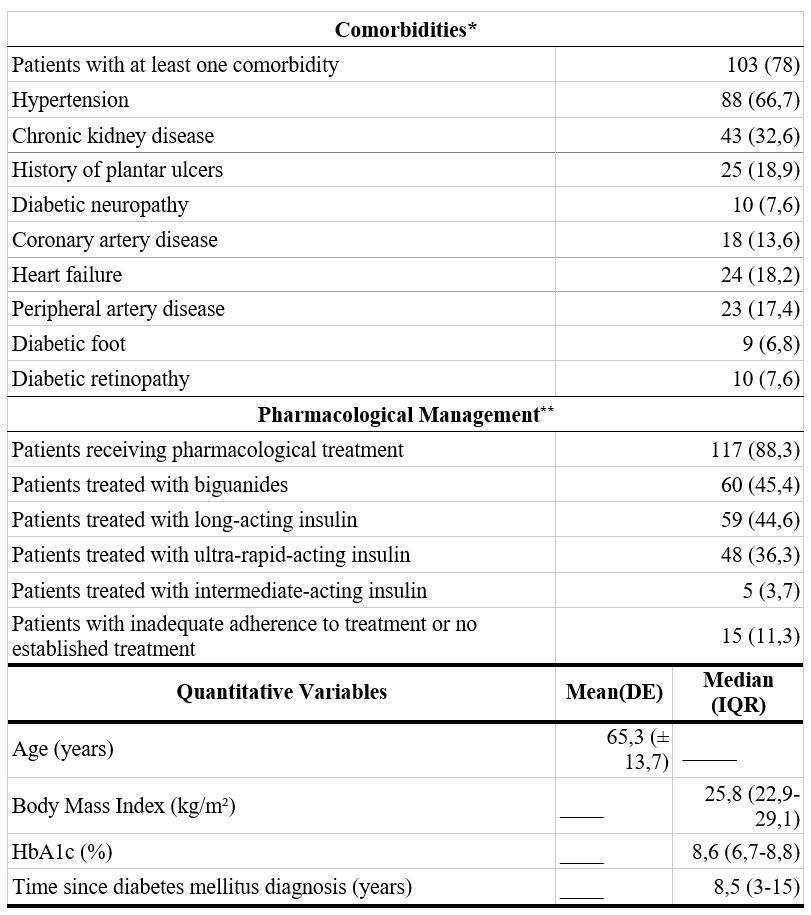
HbA1c: Glycated hemoglobin.
* Patients could present more than one comorbidity simultaneously.
** Patients could receive hypoglycemic treatment from more than one pharmacological group simultaneously.
The proportion of patients diagnosed with DPN in this study was 60.6% (n = 80; 95% CI: 0.52-0.68). No statistically significant differences were observed between men and women regarding the proportion of comorbidities (73.1% vs. 83.1%; p = 0.20), achieved metabolic control (37.3% vs. 26.2%; p = 0.19), and DPN prevalence (64.2% vs. 61.5%; p = 0.85).
Regarding positive findings in the physical examination, 68.9% of patients (n = 91) exhibited at least one abnormality during foot inspection. The most common findings included dry skin (60.6%; n = 80), hyperkeratosis (34.8%; n = 46), cracks (25%; n = 33), hammer toes (21.2%; n = 28), and prominent metatarsal bones (19.7%; n = 26). The Achilles reflex was abnormal in 60.6% of cases (n = 80), while vibratory sensitivity was impaired in 76.5% of patients (n = 101). All individuals with an active plantar ulcer (n = 22)—unilateral (68.1%; n = 15) or bilateral (22.7%; n = 7)—as well as 91.3% of patients with altered lower limb pulses (n = 23), had DPN.
Regarding the frequency of annual medical check-ups for DM, 15.9% of patients (n = 21) reported not attending any check-ups in the past year; 21.2% (n = 28) attended 1-2 check-ups; 30.3% (n = 40) attended 3-4 check-ups, and 32.6% (n = 43) attended five or more check-ups. Regarding annual podiatric check-ups, 92.4% of patients (n = 122) reported not attending any in the past year.
Concerning ulceration-amputation risk, 31.8% of individuals (n = 42) were classified as Category 1 (absence of DPN or vascular involvement); 28% (n = 37) as Category 2 (foot with signs of DPN but no vascular compromise); 18.9% (n = 25) as Category 3 (foot with signs of DPN or vascular involvement), and 21.2% (n = 28) as Category 4 (presence of a plantar ulcer).
After analyzing potential factors associated with DPN diagnosis, a statistically significant association was found with: age over 65 years, numbness in the feet, presence of physical abnormalities during podiatric inspection, abnormal Achilles reflex, and a history of lower limb arterial disease (Table 2).
Table 2.
Possible
Factors Associated with Diabetic Polyneuropathy

HbA1c:Glycated hemoglobin.
Despite the fact that the variables altered pedal pulses, history of lower limb ulcer, loss of vibratory sensation, and absence of the Achilles reflex also exhibited a statistically significant crude OR, their confidence intervals indicate imprecision due to their wide range. No association was found between the presence of DPN and variables related to educational level, sex, place of residence, body mass index, loss of glycemic control, or annual medical follow-up.
Regarding the multiple logistic regression model (Table 3), it was adjusted by including the variables that showed a statistically significant association with the DPN diagnosis. The model demonstrated good calibration, as indicated by the Hosmer-Lemeshow goodness-of-fit test (p = 0.653).
Tabla 3.
Multivariate Analysis of
Possible Factors Associated with DPN

Hosmer-Lemeshow goodness-of-fit test: p = 0,653.
Discussion
According to a multicenter study conducted in Italy, which included 816 individuals diagnosed with DM (type 1 DM: n = 123; type 2 DM: n = 693), the proportion of DPN in hospitalized patients was approximately 30.5% (20). In that study, the mean duration of DM at the time of DPN diagnosis was 16.9 years, and the average HbA1c value was 7.6%. Of all patients with type 2 DM, 4% (n = 29) were treated with insulin, 69% (n = 475) with oral medications, and 27% (n = 189) with a combination of insulin and oral medications. Regarding comorbidities associated with DM, hypertension (HTN) was the most prevalent condition (69%; n = 480). The risk factors for DPN reported in that study included being male (OR: 2.52; 95% CI: 1.8-3.56), having an elevated HbA1c value (OR: 1.36; 95% CI: 1.21-1.53), and the presence of microvascular complications (OR: 2.63; 95% CI: 1.82-3.82).
In the epidemiological study by Kisozi et al. (21), 248 diabetic patients (154 men and 94 women) hospitalized at a referral medical center in Uganda were evaluated. Among the total population, 56% (n = 140) were married; 10.9% (n = 27) were single; 19.8% (n = 49) were separated; and 12.9% (n = 42) were widowed. Additionally, 76.2% of the patients (n = 189) resided in urban areas, whereas 23.8% (n = 59) lived in rural areas. Concerning educational level, 13.7% (n = 34) of the subjects had no formal education, 54% (n = 134) had completed primary education, 24.6% (n = 61) had completed secondary education, and 7.7% (n = 19) had finished higher education. The frequency of DPN reported in that study was 29.4% (n = 73), and the risk factors associated with DPN were: age over 60 years (OR: 6.05; 95% CI: 2.08-17.63), being single (OR: 2.34; 95% CI: 1.29-4.23), a delay of more than one year in initiating hypoglycemic therapy after DM diagnosis (OR: 1.89; 95% CI: 1.03-3.39), a history of HTN (OR: 2.34; 95% CI: 1.29-4.23), and plantar ulceration (OR: 2.59; 95% CI: 1.03-6.49).
Furthermore, research conducted by Partanen et al. (22) demonstrated an increase in the frequency of DPN after 10 years of follow-up in a cohort of 133 patients newly diagnosed with DM, rising from 8.3% to 42%. The study by Andersen et al. (23) reported a cumulative incidence of DPN of 10% after 13 years of follow-up in a cohort of 1,533 diabetic patients in Denmark. Similarly, the Eurodiab IDDM study (7), which evaluated more than 3,000 diabetic patients across 16 countries, also identified a progressive increase in the proportion of DPN. Although the literature generally associates the duration of DM with an increased risk of developing DPN, no such relationship was observed in the present study (p = 0.352), which could be attributed to the methodological design employed and an insufficient sample size to detect such trends.
Regarding the most frequent findings in the physical examination, De Macedo et al. (24) reported a prevalence of dermatological disorders of approximately 51.1% in patients with DM. The most commonly observed conditions included fungal infections, xerosis, hyperkeratosis, and prurigo. In another study, Ibarra et al. (12) reported an alteration in the Achilles reflex in 31.3% and an impaired vibratory sensation in 69.5% of 348 patients with type 2 DM, findings that are similar to those observed in the present study.
Finally, the Colombian Clinical Practice Guideline for the Diagnosis and Treatment of Patients with Complicated Diabetic Foot (13) recommends that a podiatrist assess individuals classified as Category 1 for ulceration-amputation risk on an annual basis, whereas patients in Categories 2, 3, and 4 should be evaluated semiannually, quarterly, and monthly, respectively. According to the data obtained in the present study, more than 90% of individuals did not meet this recommendation in the past year.
This is alarming since non-adherence to medical treatment and inadequate follow-up can have serious implications, such as the progression of DPN, the development of complications (e.g., ulcers, infections, and non-traumatic amputations), impaired quality of life (e.g., chronic pain, loss of sensation, and difficulty performing daily activities), and an increased risk of mortality. Additionally, poor adherence to treatment and inadequate medical follow-up are associated with higher healthcare costs, due to the need for more complex treatments and prolonged hospitalizations (25).
Conclusions
The proportion of DPN in this study was higher than that reported in the reviewed literature. Additionally, a high burden of comorbidities (e.g., HTN, CKD, peripheral artery disease) was observed, along with a large number of factors associated with DPN (e.g., age > 65 years, loss of vibratory sensation and Achilles reflex, altered pedal pulses, and foot numbness). Other findings suggest poor adherence to the established recommendations for the monitoring and management of patients at risk of developing diabetic foot.
It is essential to educate patients diagnosed with DM about the importance of podiatric care to reduce the risk of ulceration and amputation, while also reinforcing the use of simple diagnostic tests. The results of this study were derived from a high-complexity institution, and they should be interpreted with caution, as they may not be representative of the general population with DM. Likewise, the clinical and demographic characteristics of the individuals included in this study may differ from those in other geographic regions. It is also important to note that the study’s duration and cross-sectional design may limit its ability to detect the long-term effects of the disease. Additional epidemiological studies are needed to determine the prevalence of DPN in the general population, with analyses tailored to the specificity of clinical and demographic variables of interest.
Acknowledgments
To the Hospital
Universitario de la Samaritana (HUS) and the Ricavta Internal Medicine Research
Group for their support in conducting this study.
References
1. Callaghan BC, Price RS, Feldman EL. Distal symmetric polyneuropathy. JAMA. 2015;314(20):2172. https://doi.org/10.1001/jama.2015.13611
2. Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol [internet]. 2012;11(6):521-34. Disponible en: https://linkinghub.elsevier.com/retrieve/pii/S1474442212700650
3. Brownlee M, Aiello LP, Cooper ME, Vinik AI, Plutzky J, Boulton AJM. Complications of diabetes mellitus. En: Williams textbook of endocrinology [internet]. Filadelfia: Elsevier; 2016. p. 1484-581. Disponible en: https://linkinghub.elsevier.com/retrieve/pii/B9780323297387000332
4. Figuerola Pino D, Reynals de Blasis E, Vidal-Puig A, Aschner Montoya P. Diabetes mellitus. En: Farreras-Rozman: medicina interna. Filadelfia: Elsevier; 2016. p. 1824-62.
5. Feldman E, Callaghan B, Pop-Busui R. Diabetic neuropathy. Nat Rev Dis Primers [internet]. 2019;5(1):42. Disponible en: https://www.nature.com/articles/s41572-019-0097-9
6. Ticse R, Pimentel R, Mazzeti P, Villena J. Elevada frecuencia de neuropatía periférica en pacientes con diabetes mellitus tipo 2 de un hospital general de Lima-Perú. Rev Med Hered. 2013 [citado 2023 jul 11];24(2):114-21. Disponible en: http://www.scielo.org.pe/pdf/rmh/v24n2/v24n2ao3.pdf
7. Tesfaye S, Stevens LK, Stephenson JM, Fuller JH, Plater M, Ionescu-Tirgoviste C, et al. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetología. 1996;39(11):1377-84. https://doi.org/10.1007/s001250050586
8. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. Retinopathy, neuropathy, and foot care: standards of care in diabetes—2023. Diabetes Care [internet]. 2023;46(Suppl_1):S203-15. Disponible en: https://diabetesjournals.org/care/article/46/Supplement_1/S203/148042/12-Retinopathy-Neuropathy-and-Foot-Care-Standards
9. Feng Y, Schlösser FJ, Sumpio BE. The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J Vasc Surg [internet]. 2009;50(3):675-682.e1. Disponible en: https://linkinghub.elsevier.com/retrieve/pii/S0741521409010283
10. Alcivar Alcivar DJ, Alvarado Cruz MS, Merchán Villafuerte KM, Journal of Scientific Research Mqri. Prevalencia de neuropatía periférica en pacientes con diabetes mellitus tipo 2. MQR Investigar [internet]. 2022;6(2):23-41. Disponible en: https://www.investigarmqr.com/ojs/index.php/mqr/article/view/41
11. Yang Z, Chen R, Zhang Y, Huang Y, Hong T, Sun F, et al. Scoring systems to screen for diabetic peripheral neuropathy. Cochrane Database Syst Rev. 2018;2018(7):CE10974. https://doi.org/10.1002/14651858.CD010974.pub2
12. Ibarra CT, Rocha JdeJ, Hernández OR, Nieves RE, Leyva R. Prevalencia de neuropatía periférica en diabéticos tipo 2 en el primer nivel de atención. Rev Med Chil. 2012;140(9):1126-31. https://doi.org/10.4067/S0034-98872012000900004
13. Asociación Colombiana de Diabetes. Guía de práctica clínica para el diagnóstico y tratamiento de los pacientes con pie diabético complicado [internet]. 2019 [citado 2023 jul 11]. Disponible en: https://asodiabetes.org/wp-content/uploads/2021/04/GuiaPractica-1.pdf?srsltid=AfmBOooE-7RVko4W273qsvrMxkKGtFHDtXE3D2E2ZlC6g0oapEE99yKn
14. Jurado J, Ybarra J, Romeo JH, Pou JM. Clinical screening and diagnosis of diabetic polyneuropathy: the North Catalonia Diabetes Study. Eur J Clin Invest. 2009;39(3):183-9. https://doi.org/10.1111/j.1365-2362.2008.02074.x
15. Didiesdle Herrera A, Sánchez Lozano A, Rodríguez Roque M, Rojas Fuentes J, Verdecia Fraga R, López Arguelles J. Evaluación electrofisiológica en pacientes diabéticos. Rev Finlay [internet]. 2017 sep [citado 2025 ene 26];7(3):187-92. Disponible en: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S2221-24342017000300005&lng=es
16. Moghtaderi A, Bakhshipour A, Rashidi H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clin Neurol Neurosurg [internet]. 2006;108(5):477-81. Disponible en: https://doi.org/10.1016/j.clineuro.2005.08.003
17. Perkins BA, Olaleye D, Zinman B, Bril V. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care [internet]. 2001;24(2):250-6. Disponible en: https://diabetesjournals.org/care/article/24/2/250/23987/Simple-Screening-Tests-for-Peripheral-Neuropathy
18. Pop-Busui R, Ang L, Boulton A, Feldman E, Marcus R, Mizokami-Stout K, et al. Diagnosis and treatment of painful diabetic peripheral neuropathy. ADA Clin Compend [internet]. 2022 [citado 2023 jul 11];2022(1):1-32. Disponible en: https://diabetesjournals.org/compendia/article/2022/1/1/147001/Diagnosis-and-Treatment-of-Painful-Diabetic
19. Herman WH, Pop‐Busui R, Braffett BH, Martin CL, Cleary PA, Albers JW, et al. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabetic Med. 2012;29(7):937-44. https://doi.org/10.1111/j.1464-5491.2012.03644.x
20. Truini A, Spallone V, Morganti R, Tamburin S, Zanette G, Schenone A, et al. A cross-sectional study investigating frequency and features of definitely diagnosed diabetic painful polyneuropathy. Pain [internet]. 2018;159(12):2658-66. Disponible en: https://journals.lww.com/00006396-201812000-00024
21. Kisozi T, Mutebi E, Kisekka M, Lhatoo S, Sajatovic M, Kaddumukasa M, et al. Prevalence, severity and factors associated with peripheral neuropathy among newly diagnosed diabetic patients attending Mulago hospital: a cross-sectional study. Afr Health Sci [internet]. 2017;17(2):463. Disponible en: https://www.ajol.info/index.php/ahs/article/view/158728
22. Partanen J, Niskanen L, Lehtinen J, Mervaala E, Siitonen O, Uusitupa M. Natural history of peripheral neuropathy in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333(2):89-94. https://doi.org/10.1056/NEJM199507133330203
23. Andersen ST, Witte DR, Dalsgaard EM, Andersen H, Nawroth P, Fleming T, et al. Risk factors for incident diabetic polyneuropathy in a cohort with screen-detected type 2 diabetes followed for 13 years: ADDITION-Denmark. Diabetes Care [internet]. 2018;41(5):1068-75. Disponible en: https://diabetesjournals.org/care/article/41/5/1068/36552/Risk-Factors-for-Incident-Diabetic-Polyneuropathy
24. de Macedo GMC, Nunes S, Barreto T. Skin disorders in diabetes mellitus: an epidemiology and physiopathology review. Diabetol Metab Syndr. 2016;8(1):63. https://doi.org/10.1186/s13098-016-0176-y
25. Silva GE, Galeano E, Correa JO. Adherencia al tratamiento: implicaciones de la no-adherencia. Acta Med Colomb [internet]. 2005 [citado 2025 enero 26];30(4):268-73. Disponible en: https://www.actamedicacolombiana.com/ojs/index.php/actamed/article/view/2469
Notes
Funding
The authors received no
funding for this research.
Conflicts of Interest
The authors declare no
conflicts of interest.
Author notes
aCorresponding author: miguelangelruizbarrera@gmail.com
Additional information
How to cite: Ruiz-Barrera MA, Cifuentes M, Boada MA, Mora JS, Bohórquez-Ballén
MC, Silva M. Proportion of Diabetic Polyneuropathy and its Possible Associated
Factors. Univ Med.
2025;66. https://doi.org/10.11144/Javeriana.umed66.ppdp