Introduction
53.9% of Egyptian women and 26% of Egyptian men are estimated to have osteopenia, the prevalence of osteoporosis in Egypt has been estimated at 28.4% in women and 21.9% in men (1). Systemic Lupus Erythematosus (SLE) is a chronic autoimmune disease characterized by dysregulated immune responses and systemic inflammation, leading to multi-organ damage (2–4). In Saudi Arabia, its prevalence is estimated at 19.28 per 100,000 individuals, with rising incidence over recent decades (5,6).
Vitamin D insufficiency is highly prevalent in SLE and may contribute to disease pathogenesis through immunomodulatory effects (7). Immune cells (B cells, T cells, antigen-presenting cells) express vitamin D receptors, enabling active vitamin D metabolites to suppress inflammatory pathways and promote tolerance (8,9). Critically, vitamin D acts as a negative acute-phase reactant: inflammation suppresses its serum levels, while immunosuppressive therapy often increases them independent of supplementation (10,11). This complicates the interpretation of low vitamin D status in active SLE.
While observational studies associate vitamin D deficiency with higher SLE disease activity, organ damage, and mortality (12-14), causal relationships remain unproven. Notably, evidence that vitamin D supplementation improves clinical outcomes (e.g., reduced flares, damage accrual, or mortality) is lacking (7,15). This represents a significant knowledge gap, as vitamin D insufficiency persists as an independent predictor of morbidity despite conventional therapy (13,14).
Therefore, this study aimed to determine the prevalence of vitamin D deficiency and insufficiency in an SLE cohort, examine relationships between circulating 25-hydroxyvitamin D, disease activity indices, and key clinical parameters, and evaluate the effects of vitamin D supplementation on immunological markers and clinical outcomes in patients with SLE.
Methods
Study Design and Setting
A retrospective cohort study was conducted at the rheumatology clinic of King Fahad Medical City Hospital in collaboration with Nourah Bint Abdulrahman University from 2016 to 2023. Ethical approval was obtained from the institutional review board (Approval Code: H-01-R-059), and the study was performed in accordance with the Declaration of Helsinki and the National Committee of Bioethics guidelines of Saudi Arabia. Written informed consent was obtained from all participants.
Participants
The study population comprised Saudi patients aged 15 years and older of both sexes who fulfilled at least four of the 1997 American College of Rheumatology revised classification criteria for SLE (16) and were receiving treatment at the KMFC rheumatology clinic during the study period. Patients with suspected malignancy and pregnant or lactating women were excluded from the analysis.
Sample size calculation
Sample size calculation was performed using G*Power
3.1.9.2 software (Universität Kiel, Germany). We conducted a pilot study with five
patients to estimate pre- and post-treatment SLEDAI scores (mean ± SD: 22.6 ± 11.8
vs. 12.6 ± 9.37). A sample size of 62 patients was calculated (effect size: 0.938,
α = 0.05, power = 95%). Accounting for a 10% dropout rate, 68 patients were enrolled.
Data collection
Clinical data were retrospectively extracted from electronic health records using a standardized data collection form. Variables collected included demographic characteristics, clinical presentation patterns, reproductive health status, disease complications and comorbidities, diagnostic investigations, and laboratory parameters at baseline and follow-up visits.
Laboratory assessments
Laboratory evaluations were conducted at pretreatment baseline and six months post-treatment initiation. Assessments included complete blood count, hepatic and renal function tests, erythrocyte sedimentation rate, C-reactive protein, urinary total protein, serum calcium levels, and 25-hydroxyvitamin D (25(OH)D) concentrations.
Serum 25-hydroxycholecalciferol vitamin D levels were quantified using the Abcam ELISA kit (catalog ab213966). Vitamin D status was categorized according to established thresholds: deficiency was defined as concentrations below 20 ng/mL, insufficiency as levels below 30 ng/mL, and normal vitamin D status as concentrations between 30 and 50 ng/mL (17). Disease activity was evaluated using the SLE Disease Activity Index (SLEDAI) score (18).
Vitamin D supplementation protocol
Patients diagnosed with vitamin D insufficiency received vitamin D3 supplementation at 8000 IU daily for four weeks, followed by maintenance therapy at 2000 IU daily. Those with vitamin D deficiency were administered 8000 IU of vitamin D3 daily for eight weeks, and subsequently continued on 2000 IU daily maintenance therapy according to established clinical guidelines. All patients received generic cholecalciferol. Post-treatment laboratory assessment was performed 6 months after initiating supplementation.
Outcome measures
The primary outcome measure was improvement in SLEDAI score following vitamin D supplementation. Secondary outcomes included the prevalence of vitamin D insufficiency and deficiency in the study population and correlation analyses between vitamin D levels and SLEDAI scores, as well as other SLE-associated clinical parameters.
Statistical analysis
Statistical analyses were performed using SPSS version 26 (IBM Inc., Chicago, IL, USA). Data distribution normality was assessed through the Shapiro-Wilk test and histogram visualization. Repeated measures analysis of variance was employed to analyze and compare means and standard deviations of continuous parametric variables. Categorical variables were analyzed using chi-square tests and presented as frequencies and percentages. Correlations between normally distributed continuous variables were examined using Pearson product-moment correlation coefficients. Statistical significance was defined as a two-tailed p-value ≤ 0.05.
Results
The mean ±SD of age was 33.5±10.46. Most participants (86.8%) were females. Out of 68 participants, 50% were married, 41.18% were single, and 8.82% were divorced. Most participants (32.4%) completed a bachelor's, and 51.47% worked remotely. Only 25% exhibited a family history of connective tissue disorder, and 4.4% disclosed a history of smoking (Table 1).
Table 1.
Demographic data
of the patients studied
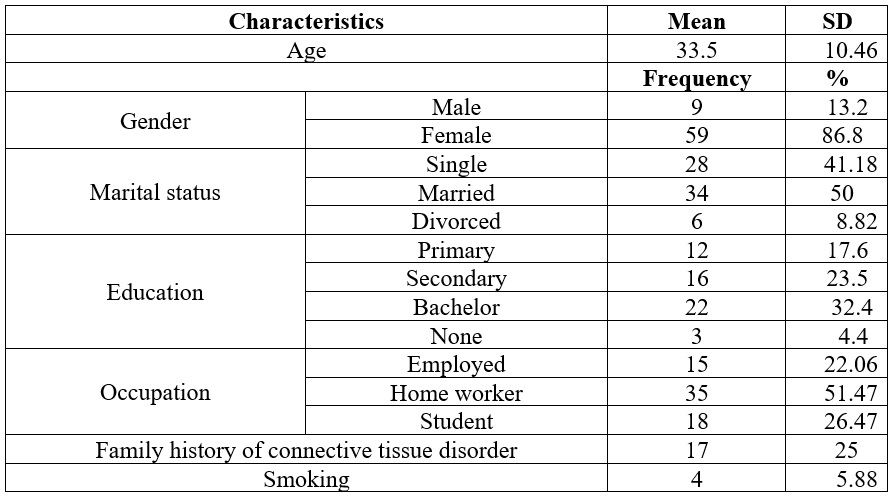
Data is presented as frequency %.
Table 2 shows that most participants (98.53%) had illness duration over six months. Fever (2.94%) and weight loss (5.9%) were uncommon. Oral ulcers appeared in 11.76%, nasal ulcers and shortness of breath in 11.8%, and photosensitivity in 60.29%. Malar rash (19.2%) and alopecia (60.3%) were frequent, while arthritis (10.29%) and arthralgia (27.94%) were the main joint issues. Menstrual irregularities affected 36.8%, with 22.1% reporting amenorrhea. OCP use was 13.24%, and 23.53% experienced pregnancy post-diagnosis, with 22.06% reporting abortions.
Table 2.
Clinical characteristics
and reproductive health of the studied patients
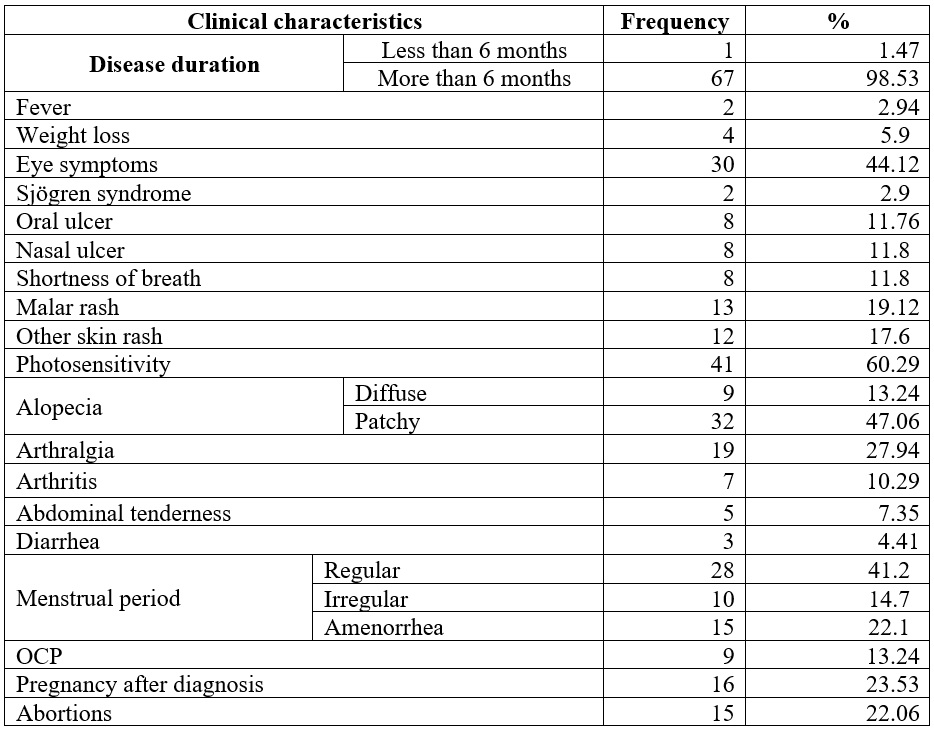
OCP: Oral contraceptive pills.
Pericarditis and pleuritic pain were found in 5.9% of patients, and pulmonary hypertension in 7.35%. IHD affected 7.4%, Raynaud’s phenomenon 22.06%, and DVT 8.8%. Colitis occurred in 4.41%, lymphadenopathy in 13.24%, and organomegaly in 8.82%. Dialysis was needed in 2.9%. Stroke affected 14.71%, mobility issues 19.12%, neuropathy 27.94%, and psychological symptoms 13.24% (Table 3).
Table 3.
Comorbidities of
the studied patients
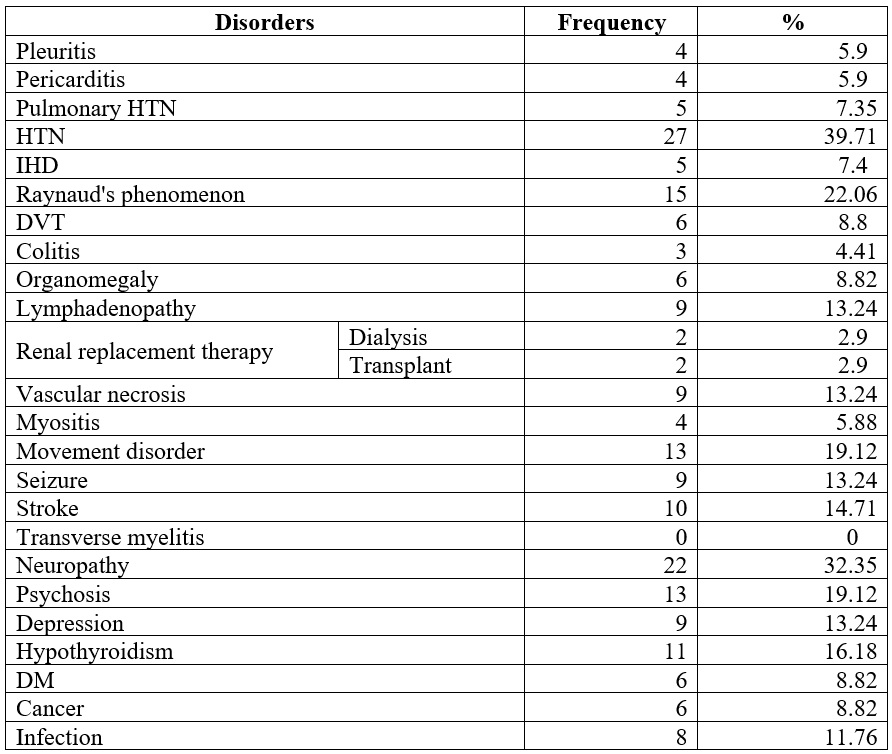
HTN: hypertension; IHD: ischemic heart disease; DVT: deep vein thrombosis; DM: Diabetes mellitus.
RBCs, albumin, vitamin D, and Ca were markedly more elevated post than pretreatment (p < 0.05). ESR, bilirubin, and ALP were substantially lower post than pretreatment (p < 0.05). Hemoglobin, RDW, WBCs, platelets, CRP, ALT, AST, Creatinine, urea, and random protein in urine were insignificantly different between pretreatment and post-treatment (Table 4).
Table 4.
Laboratory parameters
of the patients studied
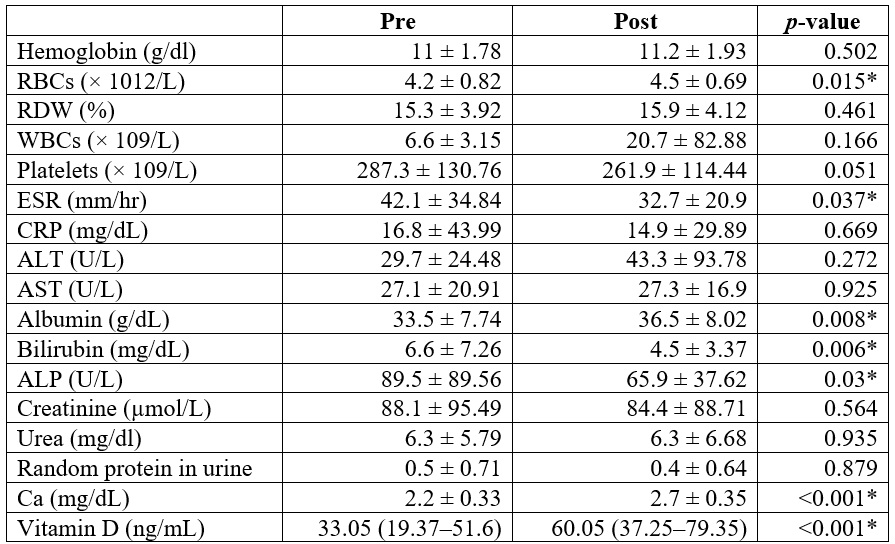
*
Significant p-value <0.05.
RBCs: red blood cell count; RDW: red cell distribution
width; WBCs: white blood cells; ESR: erythrocyte sedimentation rate; CRP: c-reactive
protein; ALT: alanine aminotransferase; AST: Aspartate aminotransferase; ALP: alkaline
phosphatase; Ca: calcium.
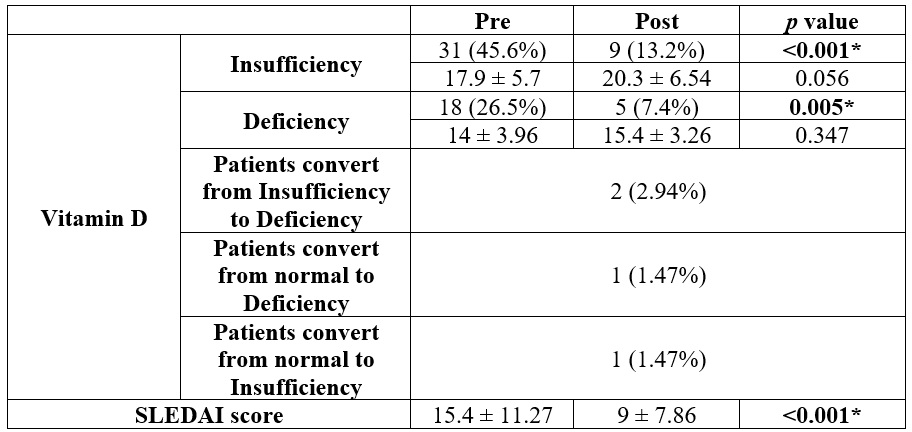
The prevalence of vitamin
D insufficiency was 31 (45.6%), and deficiency was 18 (26.5%) pretreatment, and
became 9 (13.2%) and 5 (7.4%), respectively, post-treatment. Vitamin D insufficiency
and deficiency percentage were substantially decreased post than pretreatment (p
< 0.05). SLEDAI score was considerably lower post than pretreatment (p
< 0.001) (Table 5).
Table 5.
Vitamin D insufficiency
and deficiency and SLEDAI score of the patients studied
*
Significant p-value <0.05.
SLEDAI: Systemic Lupus Erythematosus Disease Activity
Index.
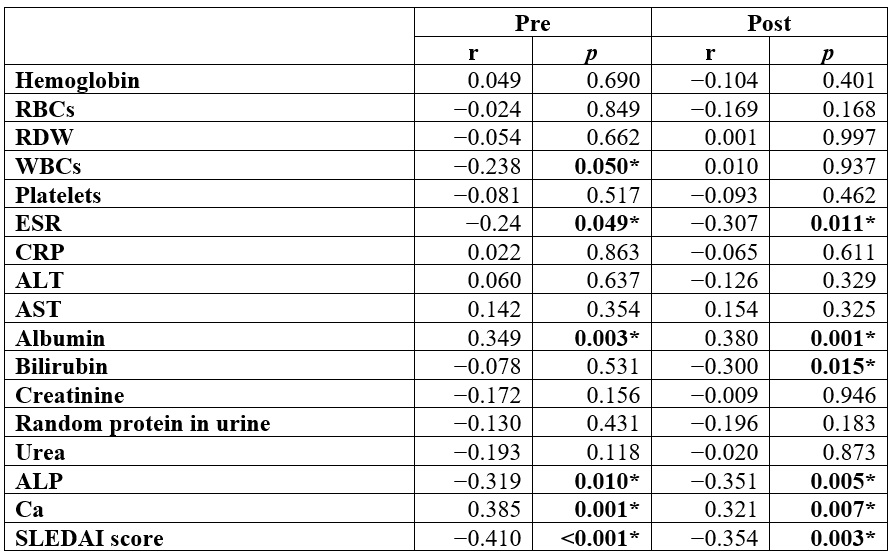
In the pretreatment
phase, vitamin D showed a substantial inverse correlation with (WBCs, ESR, ALP,
and SLEDAI score) and a positive correlation with (albumin and Ca). Post-treatment,
vitamin D showed a substantial inverse correlation with (bilirubin, ESR, ALP, and
SLEDAI score) and a substantial positive correlation with (albumin and Ca) (Table 6).
Table 6.
Correlation between
vitamin D levels and laboratory parameters of the studied patients
*
Significant p-value <0.05.
r: Pearson coefficient; RBCs: red blood cell count;
RDW: red cell distribution width; WBCs: white blood cells; ESR: erythrocyte sedimentation
rate; CRP: c-reactive protein; ALT: alanine aminotransferase; AST: aspartate aminotransferase;
ALP: alkaline phosphatase; Ca: calcium.
Discussion
Vitamin D, a steroid hormone activated in the liver and kidneys, regulates calcium metabolism (15), suppresses Th1, enhances Tregs (19,20), affects dendritic cells and IFN genes in SLE (21,22), with deficiency common from photoprotection, renal issues, and medications (23).
The study results revealed that RBCs, albumin, vitamin D, and Ca were significantly higher post than pre. ESR, bilirubin, and ALP were substantially lower post than pre. Vitamin D insufficiency and deficiency percentage were considerably lower post-treatment than pretreatment. SLEDAI score showed a substantial decrease after post-treatment in contrast to pretreatment. In the pretreatment phase, a substantial inverse correlation was observed between vitamin D and (WBCs, ESR, ALP, and SLEDAI score), while we observed a positive correlation between vitamin D and (albumin and Ca). Additionally, a substantial negative correlation was observed between vitamin D and (bilirubin, ESR, ALP, and SLEDAI score) following treatment. Conversely, a substantial positive correlation was identified between albumin, calcium, and vitamin D.
Vitamin D is essential for the immune system, as it regulates the production of antibodies, inhibits IL-2, and the proliferation of lymphocytes (24). Vitamin D3 has been found to prevent autoimmune diseases like SLE; however, its deficiency potentially leads to SLE etiology and aggravation (25). High vitamin D3 levels have been shown to lower the disease risk by 62%. Moreover, in vitro studies have shown that vitamin D3 can prevent dendritic cell differentiation, modulate T cell phenotype and function, and prevent the production of cytokines and the proliferation of T cells (26). This suggests that vitamin D status correlates with SLE disease activity through immunomodulatory mechanisms, including dendritic cell regulation and T-cell function (27). Fibromyalgia and fibromyalgianess are increased in SLE patients, and regular exercise can help improve fatigue, cognitive dysfunction, and pain from fibromyalgia (28).
Vitamin D deficiency can exacerbate SLE symptoms and increase autoantibody generation. At the same time, its immunomodulatory effects help mitigate immunological abnormalities in SLE patients, making it a risk associated with SLE disease development (29). Vitamin D is a safe and cost-effective intervention for patients with SLE, offering immune-inflammatory-modulatory benefits. It has the potential to enhance musculoskeletal and cardiovascular symptoms, preserve immune health, and prevent the excess morbidity and mortality that are associated with vitamin D deficiency (30).
In agreement with our result, Kamen et al. (31) discovered a high percentage of patients with SLE suffer from vitamin D deficiency, with substantially reduced levels of serum 25-hydroxyvitamin D. This deficiency was particularly prevalent in African Americans and individuals with photosensitivity. Similarly, Borba et al. (32) discovered that patients with high SLE activity had lower levels of vitamin D3 in comparison to controls and low-activity patients. Moreover, Bonakdar et al. (33) and Islam et al. (34) noted that at the time of diagnosis, the majority of SLE patients exhibit vitamin D deficiency, which is correlated with an increased clinical activity. Furthermore, Mahmoud et al. (35) demonstrated that the correlation between vitamin D and (ESR and ALP) was inverse, while Ca and serum albumin exhibited a positive correlation with vitamin D. However, no correlation was observed between vitamin D and WBCs in patients with SLE. The correlation between vitamin D and (ESR and ALP) was inverse, while Ca and serum albumin exhibited a positive correlation with vitamin D. However, no relationship was observed between vitamin D and WBCs in patients with SLE. Also, Acosta Colman et al. (36) showed that there was an inverse relationship between vitamin D concentrations and SLE disease activity.
Our trial has several limitations, including a relatively limited sample size and single-center design, which limits generalizability to broader populations. The retrospective nature of the study prevented standardized follow-up intervals and comprehensive documentation of concurrent medications that could influence both vitamin D metabolism and SLE activity. Detailed information on concurrent SLE treatments, including immunosuppressive medications, corticosteroids, and other therapeutic interventions, was not systematically available, limiting our ability to isolate the independent effects of vitamin D supplementation. Additionally, data on patient adherence to supplementation protocols, baseline dietary vitamin D intake, and sun exposure patterns were not collected, which could have influenced the observed outcomes. Further prospective, controlled trials with standardized treatment protocols are needed to establish definitive guidelines for vitamin D supplementation in SLE management and to confirm the causal relationship between vitamin D status and disease activity improvement.
Conclusions
The prevalence of vitamin D insufficiency was 31
(45.6%), and deficiency was 18 (26.5%) pretreatment, and became 9 (13.2%) and 5
(7.4%), respectively, post-treatment. The results indicated a substantial correlation
between vitamin D levels and (bilirubin, ESR, ALP, albumin, and Ca). Vitamin D deficiency showed an inverse correlation with SLE
activity before treatment, while vitamin D supplementation correlated with reduced
SLE activity.
References
1. El Miedany Y, El Gaafary M, Gadallah N, Mahran S, Abu-Zaid MH, Hassan W, et al. Screening to prevent osteoporotic fractures in Egypt: a position statement of the Egyptian Academy of Bone Health. Egypt Rheumatol Rehabil. 2024;51:8. https://doi.org/10.1186/s43166-024-00240-1
2. Fanouriakis A, Tziolos N, Bertsias G, Boumpas DT. Update οn the diagnosis and management of systemic lupus erythematosus. Ann Rheum Dis. 2021;80:14-25. https://doi.org/10.1136/annrheumdis-2020-218272
3. Pan L, Lu M-p, Wang J-H, Xu M, Yang S-R. Immunological pathogenesis and treatment of systemic lupus erythematosus. World J Clin Pediatr. 2020;16:19-30. https://doi.org/10.1007/s12519-019-00229-3
4. Zharkova O, Celhar T, Cravens PD, Satterthwaite AB, Fairhurst A-M, Davis LS. Pathways leading to an immunological disease: systemic lupus erythematosus. Rheumatology. 2017;56:55-66. https://doi.org/10.1093/rheumatology/kew427
5. Fava A, Petri M. Systemic lupus erythematosus: diagnosis and clinical management. J Autoimmun. 2019;96:1-13. https://doi.org/10.1016/j.jaut.2018.11.001
6. AlOmair M, AlMalki H, AlShahrani M, Mushait H, Al Qout M, Alshehri T, et al. Clinical manifestations of systemic lupus erythematosus in a tertiary center in Saudi Arabia. Cureus. 2023;15:12-23. https://doi.org/10.7759/cureus.41215
7. Gergianaki I, Fanouriakis A, Repa A, Tzanakakis M, Adamichou C, Pompieri A, et al. Epidemiology and burden of systemic lupus erythematosus in a southern european population: Data from the community-based lupus registry of Crete, Greece. Ann Rheum Dis. 2017;76:1992-2000. https://doi.org/10.1136/annrheumdis-2017-211206
8. Yeoh S-A, Dias SS, Isenberg DA. Advances in systemic lupus erythematosus. Medicine. 2018;46:84-92. https://doi.org/10.1016/j.mpmed.2017.11.010
9. Siegel CH, Sammaritano LR. Systemic lupus erythematosus: a review. JAMA. 2024;331:1480-91. https://doi.org/10.1001/jama.2024.2315
10. Elkon K, Casali p. Nature and functions of autoantibodies. Nat Clin Pract Rheumatol. 2008;4:491-8. https://doi.org/10.1038/ncprheum0895
11. Ludwig RJ, Vanhoorelbeke K, Leypoldt F, Kaya Z, Bieber K, McLachlan SM, et al. Mechanisms of autoantibody-induced pathology. Front Immunol. 2017;8:603-21. https://doi.org/10.3389/fimmu.2017.00603
12. Mackern-Oberti JP, Vega F, Llanos C, Bueno SM, Kalergis AM. Targeting dendritic cell function during systemic autoimmunity to restore tolerance. Int J Mol Sci. 2014;15:16381-417. https://doi.org/10.3390/ijms150916381
13. Sakthiswary R, Raymond AA. The clinical significance of vitamin D in systemic lupus erythematosus: a systematic review. PLoS One. 2013;8:552-75. https://doi.org/10.1371/journal.pone.0055275
14. Wimalawansa SJ. Infections and autoimmunity-the immune system and vitamin D: a systematic review. Nutrients. 2023;15:3842-56. https://doi.org/10.3390/nu15173842
15. Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020;12:20-97. https://doi.org/10.3390/nu12072097
16. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. https://doi.org/10.1002/art.1780400928
17. Al-Alyani H, Al-Turki HA, Al-Essa ON, Alani FM, Sadat-Ali M. Vitamin D deficiency in Saudi Arabians: a reality or simply hype. A meta-analysis (2008-2015). J Family Community Med. 2018;25:1-4. https://doi.org/10.4103/jfcm.JFCM_73_17
18. Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288-91.
19. Bikle DD. Vitamin D regulation of immune function. Curr Osteoporos Rep. 2022;20:186-93. https://doi.org/10.1007/s11914-022-00732-z
20. Wimalawansa SJ. Physiology of vitamin D-focusing on disease prevention. Nutrients. 2024;16:1666-92. https://doi.org/10.3390/nu16111666
21. Wu H, Chang C, Lu Q. The epigenetics of lupus erythematosus. In: Chang C, Lu Q, editors. Epigenetics in allergy and autoimmunity: advances in experimental medicine and biology. Vol. 1253. Singapore: Springer; 2020. https://doi.org/10.1007/978-981-15-3449-2_7
22. Nerviani A, Mauro D, Gilio M, Grembiale RD, Lewis MJ. To supplement or not to supplement? The rationale of vitamin D supplementation in systemic lupus erythematosus. Open Rheumatol J. 2018;12:226-47. https://doi.org/10.2174/1874312901812010226
23. Hassanalilou T, Khalili L, Ghavamzadeh S, Shokri A, Payahoo L, Bishak YK. Role of vitamin D deficiency in systemic lupus erythematosus incidence and aggravation. Auto Immun Highlights. 2017;9:1-5. https://doi.org/10.1007/s13317-017-0101-x
24. Skrobot A, Demkow U, Wachowska M. Immunomodulatory role of vitamin D: a review. In: Pokorski M, editor. Current trends in immunity and respiratory infections. Vol. 1108. Cham: Springer; 2018. p. 13-23. https://doi.org/10.1007/5584_2018_246
25. Yamamoto E, Jørgensen TN. Immunological effects of vitamin D and their relations to autoimmunity. J Autoimmun. 2019;100:7-16. https://doi.org/10.1016/j.jaut.2019.03.002
26. Bscheider M, Butcher EC. Vitamin D immunoregulation through dendritic cells. Immunology. 2016;148:227-36. https://doi.org/10.1111/imm.12610
27. Dutta C, Kakati S, Barman B, Bora K. Vitamin D status and its relationship with systemic lupus erythematosus as a determinant and outcome of disease activity. Horm Mol Biol Clin Investig. 2019;38:20-64. https://doi.org/10.1515/hmbci-2018-0064
28. Battesti G, Felten R, Piga M, Sarmiento-Monroy JC, Ziade N, El Kibbi L, et al. Prevalence and characteristics of, and knowledge related to, photosensitivity in patients with lupus erythematosus: the international PHOTOLUP study. Rheumatology. 2023;1:5-48.
29. Colotta F, Jansson B, Bonelli F. Modulation of inflammatory and immune responses by vitamin D. J Autoimmun. 2017;85:78-97. https://doi.org/10.1016/j.jaut.2017.07.007
30. Utami AT. Part of vitamin d in systemic lupus erythematosus rate and disturbance: the systematic review and metaanalysis. Biomed J Sci Tech Res. 2022;42:33939-46. https://doi.org/10.26717/BJSTR.2022.42.006801
31. Kamen DL. Vitamin D in lupus: new kid on the block? Bull Hosp Jt Dis. 2010;68:218-35.
32. Borba V, Vieira J, Kasamatsu T, Radominski S, Sato E, Lazaretti-Castro M. Vitamin D deficiency in patients with active systemic lupus erythematosus. Osteoporos Int. 2009;20:427-33. https://doi.org/10.1007/s00198-008-0676-1
33. Bonakdar Z, Jahanshahifar L, Jahanshahifar F, Gholamrezaei A. Vitamin D deficiency and its association with disease activity in new cases of systemic lupus erythematosus. Lupus. 2011;20:1155-60. https://doi.org/10.1177/0961203311405703
34. Islam MA, Khandker SS, Alam SS, Kotyla p, Hassan R. Vitamin D status in patients with systemic lupus erythematosus (SLE): a systematic review and meta-analysis. Autoimmun Rev. 2019;18:102-392. https://doi.org/10.1016/j.autrev.2019.102392
35. Mahmoud HM, Elhendy YA, Amr GE, Elzwawy RAE, Kamal NM. Vitamin D pattern in patients with systemic lupus erythematosus with and without nephritis. Egypt J Hosp Med. 2021;85:2695-26700. https://doi.org/10.21608/ejhm.2021.189618
36. Acosta-Colman I, Morel Z, Paats A, Ortíz N, Román L, Vázquez M, et al. Association between vitamin D deficiency and disease activity in Paraguayan patients with systemic lupus erythematosus. Rev Colomb Reumatol. 2022;29:19-25. https://doi.org/10.1016/j.rcreue.2020.12.001
Notes
Conflict of interests:
The author(s) declare
no conflict of interest.
Funding:
No funding was received to conduct this study.
Acknowledgment:
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R782), Princess Nourah bint Abdulrahman University,Riyadh,Saudi Arabia.
Author notes
a Correspondence author: dmismail@pnu.edu.sa
Additional information
How to cite: Ismail D, Bakhsh E, Qushmaq K, Alfraijy N, Alhabardi H, Alzazmi R, Alghmdi D. The effect of vitamin d level on systemic lupus erythematosus in Saudi patients at a tertiary care center. Univ Med. 2025;66. https://doi.org/10.11144/Javeriana.umed66.evls