INTRODUCTION
In the last decades, dental implants have become the treatment of choice to rehabilitate partially or totally edentulous patients, due to their predictability and long-term success. However, even when planning and placement are performed under strict quality controls, complications may arise during the surgical procedure, rehabilitation, or maintenance. During the implant maintenance stage, inflammatory complications can arise, which, if not treated correctly, can progress and lead to implant loss. These inflammatory complications are known as peri-implant diseases and include peri-implant mucositis and peri-implantitis (1-4).
Despite the high rates of implant survival reported in the literature (1,2), up to 92.6 % complications can occur leading to partial or total loss of osseointegration, therefore, their failure. Important to consider is that the survival of an implant does not mean it is successful. Implant survival is defined as permanence in the mouth, regardless of the surrounding conditions. Defining implant success is more challenging as it depends on the success criteria used, such as mobility, marginal bone loss, radiolucency around the implant surface, symptoms reported by the patient, recurrent infections, and satisfactory aesthetics for the patient and the clinician (3,4). An implant fails when it loses osseointegration and must be extracted.
The failure of an implant can happen at any time, from the moment of its placement, when good primary stability is not obtained (mechanical anchoring of the implant in the bone), to when it has already been rehabilitated, where various conditions can produce a loss of bone-implant contact (BIC) and loss of osseointegration. Failures occurring before the prosthetic rehabilitation of the implant happen earlier and those occurring on the implant-supported restoration happen later. Failures are due to mechanical complications on the implant, its components, or the restoration, while biological complications affect the peri-implant tissues (5).
The Consensus Report of the Sixth European Workshop on Periodontology (6) defines peri-implant mucositis as a reversible inflammatory reaction with erythema and inflammation of the peri-implant mucosa, bleeding or suppuration on probing, and increased probing depth (4-5 mm). Peri-implantitis is defined as an inflammatory process that affects the hard and soft tissues surrounding an osseointegrated implant, resulting in loss of bone support around the implants. This is often associated with increased probing depth (< 5mm) and bleeding and/or suppuration on probing (7,8).
Peri-implant mucositis is characterized by bleeding on probing, erythema, inflammation, or suppuration. Due to inflammation and decreased tissue resistance, an increase on probing is usually seen. In peri-implantitis there is inflammation of the peri-implant tissues and progressive loss of the bone supporting the implant (radiographically proven), presence of bleeding on probing or suppuration, increased probing depth, or recessions (9,10).
Risk factors of peri-implantitis are chronic periodontitis, poor oral hygiene, and lack of periodontal maintenance. Regarding diabetes and smoking as risk factors, data are not conclusive. Evidence available is not enough to establish if excessive cement at the submucosal level, little keratinized mucosa, and difficulty in performing adequate hygiene due to the position of the implant are risk factors of peri-implantitis (11)
Linkevicus, et al. (12) report that cemented implant-supported restorations present a risk factor for the development of peri-implant disease, mainly in patients with a history of periodontal disease. The excess of subgingival cement is a retention factor for biofilm, which produces peri-implant inflammation. The closer the cementum is to the bone crest, the greater the risk of developing peri-implantitis.
In addition, the presence of little keratinized tissue is frequently observed in implant therapy associated with a low height of the alveolar ridge. It is considered that the keratinized mucosa works as a barrier for the implants, preventing recessions and favoring the control of biofilm, and there should be a band of at least 2 mm wide around the teeth. Ideally, this keratinized gum band should be created before placing the implants (7).
Studies on the prevalence of peri-implant disease are limited by the lack of universal diagnostic criteria. There are studies on the prevalence of peri-implant disease in the literature, such as the one by Derks and Tomasi (13) who reported an average of 43 % peri-implant mucositis and 22 % peri-implantitis. Atieh, et al. (14) report a 18.8 % prevalence of peri-implantitis, and other authors suggest that one in five patients will present peri-implant disease five or more years after implant placement (15).
Knowing the frequency of peri-implant diseases and the risk factors that can be considered to prevent them, provides knowledge to the professional so that, when placing implants and afterwards, complications that may arise are avoided as much as possible. Additionally, such studies are necessary to provide evidence required in the quality analysis of patient care for a relatively recently implemented procedure such as dental implantology. Therefore, the research question of this study was: What is the frequency of peri-implant diseases and possible associations with potential risk factors in patients treated at the clinics of the Pontificia Universidad Javeriana’s Dental School (Bogotá, Colombia)?
MATERIALS AND METHODS
Prior approval by the Research and Ethics Committee of the Pontificia Universidad Javeriana’s Dental School, this retrospective observational study was conducted with the population of patients who had received dental implants between 2008 and 2015 at the School.
The initial sampling was carried out from a database of patients seen in the Dental School’s graduate clinics. Clinical records of 18-year-old patients over older were included, completed with other data collected for the study, and who had an initial X-ray taken at the time of the implant placement or one month later at most. Patients with distorted radiographs, defined by differences between the actual length of the implant and the length reported in the clinical history of more than 25 % were excluded. Once the records were selected, eligible patients were contacted by telephone. They were asked to make an appointment for maintenance and assessment and maintenance of implants placed at the School, emphasizing the importance of maintenance to prevent peri-implant diseases and implant loss. Patients who were unreachable by phone received emails.
An instrument was used to collect data from the clinical records: date of implant placement, implant dimensions, location of the implant, type of prosthesis, use of previous bone graft or in time of implant placement, type of bone graft, type of restoration (cement-retained or screw-retained), existing systemic diseases, history of periodontal disease, smoking, age, gender, oral hygiene habits, radiographic height of the bone crest in mesial and distal to the implant, and amount of keratinized tissue in the implant area. During the maintenance appointment, the following data were recorded: current health status, smoking, existing systemic diseases, periodontal probing depth, bleeding on probing, implant mobility, radiographic bone changes with respect to initial X-ray, presence of periodontal disease, dental plaque index, amount of keratinized tissue in the implant area, symptoms, and suppuration.
The diagnosis used the criteria defined at the Consensus Report of the 6th European Workshop on Periodontology. To diagnose mucositis, the presence of bleeding on probing, inflammation of the soft tissues around the implant, and < 2 mm radiographic bone loss when compared to the initial radiograph were recorded. To diagnose peri-implantitis, the following signs were collected: > 5 mm probing depth, > 2 mm radiographic bone loss when compared to the initial radiograph, and presence of bleeding on probing.
In the clinical assessment, a Hu-Friedy® PCP-UNC15 periodontal probe was used to determine the probing depth. Six measurements were recorded per implant present, in millimeters, on the mesial, medial and distal surfaces on buccal and the same on lingual/palatal. Bleeding on probing was measured as a binary variable (presence or absence) for each of the implant surfaces, these being: mesial, medial and distal for vestibular and the same for lingual/palatal.
For the radiographic assessment, the same type of radiograph was taken as the initial one stored in the clinical record (panoramic or periapical). For the periapical radiographs, parallelism technique was used with the help of an XCP brand radiograph positioning system. To determine the percentage of bone loss, the bone height mesial and distal to the implant in the current radiograph was compared with the radiograph taken after implant placement (less than one month). The bone height reference was considered to be that corresponding to the control radiograph after implant placement.
Researcher calibration was performed to ensure inter-examiner reliability. This was carried out by completing the examination of the first five participants by the two student examiners of the graduate program in Periodontics of the Pontificia Universidad Javeriana and a third specialist examiner in the area. The examiners evaluated all radiographs of the participants independently. If there was a difference between any of the determined measures, an additional joint examination was conducted to try to reach an agreement. If this consensus was not reached, the radiographs were evaluated by a fourth examiner.
Data Analysis
The data analysis was performed both at the patient and implant levels (for sample characterization), in which the frequency of peri-implant diseases in both groups was estimated. A patient was counted as a case of mucositis if one or more of her implants met the diagnostic criteria for mucositis. Likewise, a patient was considered a case of peri-implantitis when one or more of their implants met the peri-implantitis criteria. The same patient could belong to both groups. Patients were categorized as healthy if they did not present any peri-implant diseases. To determine the possible associations between peri-implant diseases both at the patient and implant levels and their potential risk factors, a logistic regression analysis was performed, whose outcome or response variable was healthy or unhealthy. All analyzes were performed at a 95 % confidence level.
RESULTS
Participants in the Study
A total of 75 patients were assessed for this study, of whom 52 (69.3 %) were females and 23 (30.7 %) males, aged between 21 and 82 years with an average age of 59.7 years, and a standard deviation of 11.6 years. About 50 % were over 60 years old. Seven (9.3 %) patients reported smoking, 10 (13.3 %) had bruxism, and 1 (1.3 %) was an oral breather. The most frequent comorbidity was hypothyroidism, which corresponded to 45.4 % of the patients who presented some systemic disease, followed by arterial hypertension in 42.4 % (Table 1).
318 implants were found in the 75 patients of the study, ranging from 1 to 10 implants and an average of 4 implants per patient. Regarding the type of restoration, 157 implants were restored with individual crowns (49.3 %), 93 with hybrid prostheses (29.2 %), 52 with fixed implant-supported prostheses (16.3 %), 4 with provisional prostheses (1.3 %), and 12 with implant-supported removable prostheses (overdenture) (3.8 %).
TABLE 1
Characteristics of the Population Studied
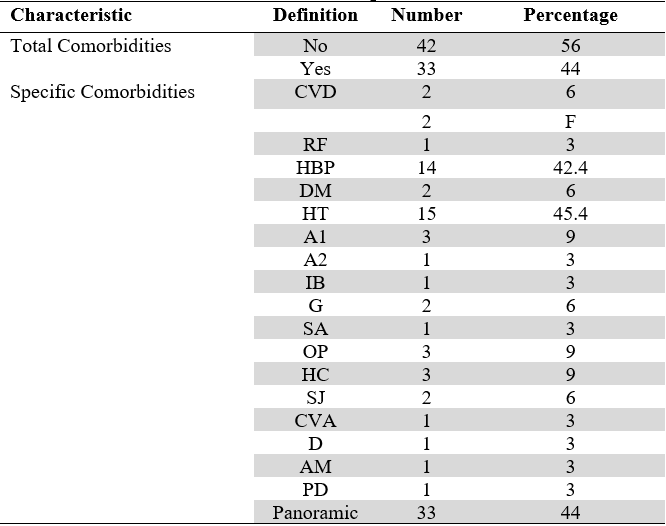
CVD: Cardiovascular disease; HBP: High Blood Pressure; HT: Hypothyroidism; DM: Diabetes Mellitus; A1: Arthrosis; A2: Arthritis; SA: Sleep Apnea; RF: Rheumatic Fever; AM: Asthma; G: Gastritis; IB: Irritable Bowel; O: Osteopenia; HC: Hypercholesterolemia; OP: Osteoporosis; SJ: Sjögren’s Syndrome; F: Fibromyalgia; CVA: Cerebrovascular Accident; D: Depression; PD: Pre-Diabetes.
Source: the authors.
Peri-Implant Diseases
The total percentage of peri-implant diseases was 81.3 % patients and 76.3 % implants. The frequency of peri-implant mucositis and peri-implantitis was 74.7 % and 34.7 % respectively, at the patient level, and 55.7 % and 20.7 % respectively at the implant level. 21 of the patients had both mucositis and peri-implantitis in their implants. 35 patients had at least one implant with mucositis, but none with peri-implantitis, and five patients had implants with peri-implantitis, but none with mucositis.
Of the 318 implants, 0.7 % were placed in 2008, 2.3 % in 2009, 3.9 % in 2010, 15.3 % in 2011, 21.8 % in 2012, 28.3 % in 2013, 7.8 % in 2014 and 19.9 % in 2015. The percentage of peri-implant disease per year was variable and ranged from 58.3 % to 100 % (Figure 1).
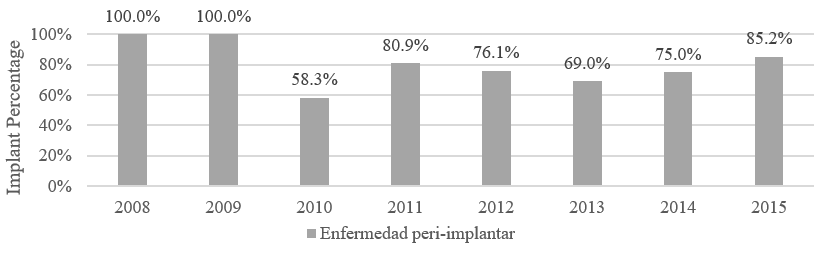 FIGURE 1
Frequency of Peri-implant Diseases According to Year of Placement of Dental Implants
FIGURE 1
Frequency of Peri-implant Diseases According to Year of Placement of Dental Implants
Source: the authors.
The analysis of the relationship between peri-implant disease and the variables of gender and age showed that all patients under 49 years of age in the study had some type of peri-implant disease. When analyzing the association of peri-implant disease among the patients according to their age group, presence of comorbidities and gender, no appreciable differences were found, so it was expected that they would not be significant variables in the model (Figure 2).
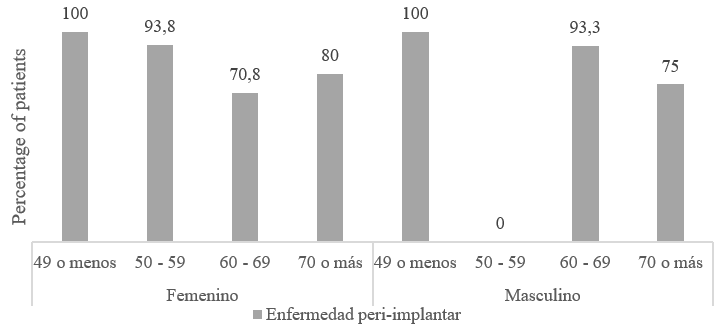 FIGURE 2
Frequency of Peri-implant Disease According to Sex and Age
FIGURE 2
Frequency of Peri-implant Disease According to Sex and Age
Source: the authors.
When asked about smoking, only seven patients reported presenting this habit, which represents 9.3 % of the sample. A total of 23 implants were placed in these patients, of which 91.4 % had a diagnosis of peri-implant disease. On the other hand, 10 patients reported having bruxism and one suffered from oral breathing. In the latter patient peri-implant disease was found in their nine implants. A total of 48 implants were assessed in patients with bruxism, of which 37 (77 %) were diagnosed with peri-implant disease.
When analyzing the relationship between periodontal disease and the development of peri-implant disease, patients with a history of periodontal disease showed a high frequency of peri-implant disease (82.4 %). Among patients with current periodontal disease, 83.3 % had at least one implant with peri-implant disease.
The amount in millimeters of keratinized mucosa around the implants was assessed in order to relate it to peri-implant disease. In the groups with the greatest amount of keratinized gingiva around the implant (5-6 mm), 100 % of peri-implant disease was found, unlike the lowest values of keratinized gingiva (2-3 mm) whose percentages were 66.15 % and 59.7 %. %, respectively.
Due to the different diameters of implants placed, which depended on the brand, diameter ranges were created to facilitate the presentation and analysis of results. An increasing frequency of peri-implant disease was observed with larger implant diameters (Figure 3).
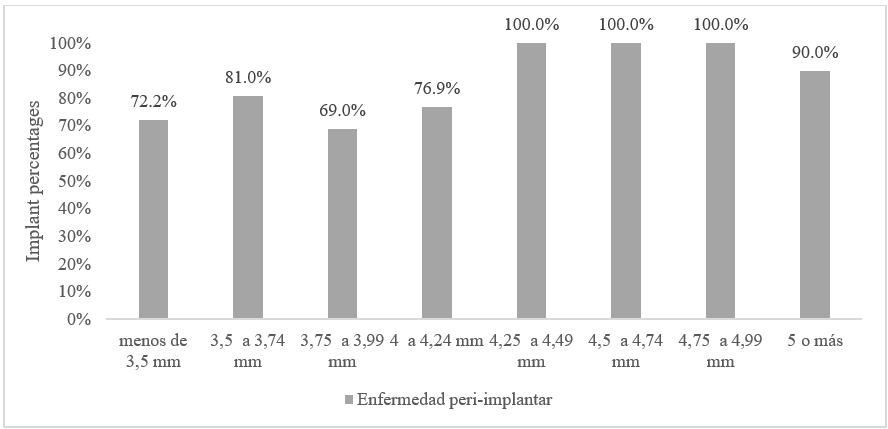 FIGURE 3
Frequency of Peri-implant Disease according Implant Diameter
FIGURE 3
Frequency of Peri-implant Disease according Implant Diameter
Source: the authors.
Regarding the frequency of peri-implant disease according to the type of rehabilitation, 3.77% (12 implants) were type “S.” Of those, 100% presented peri-implant disease. No appreciable differences were observed between the proportion of healthy patients according to the type of rehabilitation (Figure 4).
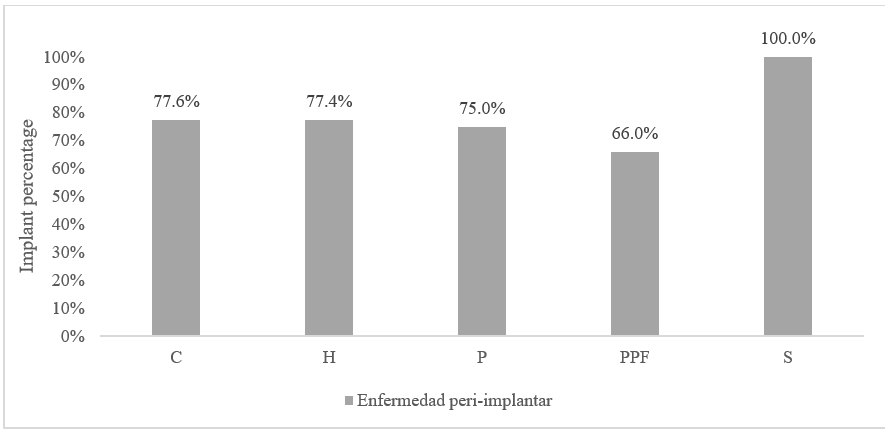 FIGURE 4
Frequency of Peri-implant Disease According to Type of Rehabilitation
FIGURE 4
Frequency of Peri-implant Disease According to Type of Rehabilitation
Source: the authors.
Binomial Logistic Model
As a multivariate procedure that would reveal the main factors associated with the presence of peri-implant disease, a multivariate logistic model was created, whose dependent variable was having or not peri-implant disease. The models were run at two levels, patient level and implant level. Taking a value of p<0.05 as significant, the following variables for patients were included in the final model: age, presence of hypothyroidism, history of periodontal disease, and total number of implants. For the analysis by implants, additionally, significance was found for the deepest vestibular and palatal probing, the amount of attached gingiva, and the type of rehabilitation (Table 2).
Variables with p-value close to 0 and in general up to 0.1 were significant for the models. To make the fact more illustrative, those that had stars and by increasing their number of stars, significantly modified the chance of presenting peri-implant disease. In general, it is assumed that variables without statistical significance should be removed from the models. Additionally, it is important to highlight that variables were excluded due to violation of assumptions, since the most important of them was that there was no correlation between the independent variables. Thus, multicollinearity within the models was avoided.
TABLE 2
Anova for Binomial Regression Trimmed Model for Implants
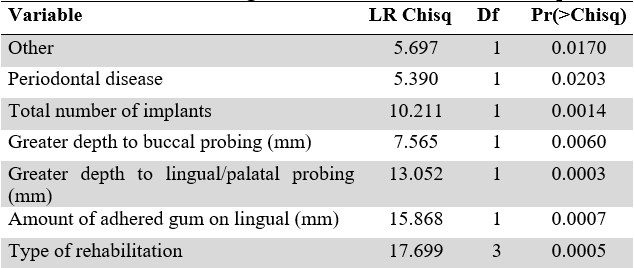
Source: the authors.
To arrive at the final models proposed in this analysis, confusion diagnoses were made on the variables, in order to show the true association between the covariates and the dependent variable. This explains the fact of having left in the model variables that, as can be seen, were not statistically significant. Table 3 shows the crossover between the prediction made with the processed trimmed models and the true results.
TABLE 3
Classification from the trimmed binomial regression model
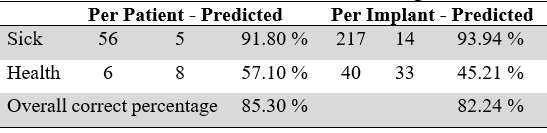
Source: the authors.
An average predictive power of 74.45 % for the patient model and 70 % for the implant model was established, resulting from the average of the success percentages in each of the categories of the response variable. The overall correct percentage is calculated from the sum of patients/implants correctly classified over the total number of patients/implants. However, this measurement can generate high and wrong conclusions of success. The threshold for classifying a patient/implant in the categories of diseased or healthy was 50%, that is, if the model yields an estimated probability greater than 50 % of presenting peri-implant disease, the patient/implant is classified as sick. This model, despite it predicts the fact of presenting peri-implant disease in an ideal way (Chi square P-value between the model’s prediction and the real result for patients = 0.000. Chi square P-value between the model’s prediction and the real result for implants = 0.034). It should be noted that the main goal of these models was to establish the characteristics associated with presenting peri-implant disease. For this reason, the conclusions focus on the odds ratio table.
TABLE 4
Odds Ration-based Protection and Risk Factors Identified for the Peri-implant Disease Outcome
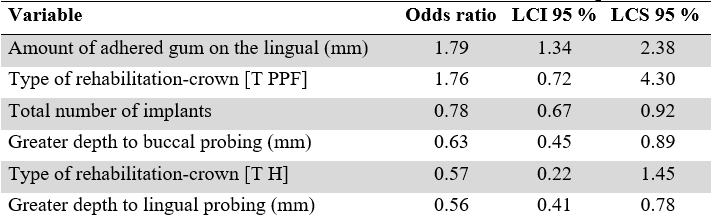
Source: the authors.
Table 4 shows the odds ratio and their 95 % confidence intervals. They are the logistic regression measures that do offer an interpretation, for example: the probability that an implant presents peri-implant disease decreases to 0.57 times, if the type of rehabilitation-crown is hybrid. In short, odds ratio > 1 are related to having a higher probability of presenting peri-implant disease, that is, it would be considered a risk factor. In contrast, the odds ratio < 1, represent a lower probability of presenting the outcome.
DISCUSSION
Currently, peri-implant diseases are controversial regarding their diagnosis and treatment. For this reason, knowing the frequency of the pathology and identifying its risk factors in a population will allow carrying our correct planning and implementation. In addition, such knowledge will allow to develop prevention, maintenance, and treatment programs for these pathologies in high and low risk groups.
Worldwide, multiple studies have been conducted to determine the frequency of peri-implant disease. According to different reports in the literature, the frequency has been increasing over time; however, the main problem with the studies has been the inconsistency in definitions, reporting methods, and research designs to establish the prevalence of peri-implant disease. An exhaustive search was carried out in which studies published in previous years and currently were identified.
Derks, et al. (16), in a sample of 588 randomly selected patients, who had received treatment with implants nine years before, performed a clinical and radiographic analysis. They evaluated the prevalence of peri-implantitis and identified indicators of risk using multivariate regression analysis. 45 % of all patients had peri-implantitis (bleeding on probing/suppuration and bone loss > 0.5 mm). In addition, moderate/severe peri-implantitis (bleeding on probing/suppuration and bone loss >2 mm) was diagnosed in 14.5 % of cases.
Atieh, et al. (14) carried out a systematic review and meta-analysis in 2012 with studies obtained from electronic databases. They identified 504 studies with 1497 participants and 6383 implants. The estimated frequency of peri-implant mucositis was 63.4 % participants and 30.7 % implants, while the prevalence of peri-implantitis was 18.8 % in participants and 9.6 % among implants. It occurred more frequently in smokers with an approximate estimate of 36.3% cases.
In a multicenter cross-sectional study published in 2017 in Japan, Ogata, et al. (17) investigated the prevalence of peri-implant diseases in adults who attended follow-up appointments for maintenance procedures. That study included 267 patients (110 men and 157 women with a mean age of 62.5 years) who had at least three years of loading time. The tissues around the implant were examined and classified into peri-implant mucositis and peri-implantitis, the former being defined as “mild” bleeding on probing (<25N) and the latter defined as changes in the crestal bone with bleeding on probing. They determined a prevalence of healthy peri-implant tissue in 57%, peri-implant mucositis in 33.3% and peri-implantitis in 9.7%, having used Nobel Replace, Straumann and Brânemark implants with different surfaces, which the author considers could have influenced the prevalence. Peri-implantitis rates of 23.53% in smokers and 16.51% in non-smokers were reported.
Recently, a cross-sectional study carried out on patients at a university clinic showed that the prevalence of peri-implantitis was 56.6 % among patients and 27.9% among implants (18). Such values are higher than those found in our study, considering that it was also carried out in university dental clinics.
In Latin America, specifically in Colombia, there is little literature on peri-implant diseases, we found a prevalence study in 64 implants and 25 patients that considered clinical variables such as pocket depth, bleeding on probing, present plaque, mobility, gingival recession, attachment loss and radiographic bone loss. The presence of a pocket > 5 mm with hemorrhage and bone loss > 2 mm was used to define of peri-implantitis. One year after implant loading, the prevalence of peri-implant mucositis and peri-implantitis was 81.2 % and 15.6 %, respectively, a prevalence that was lower in implants with a reduced platform (19).
There is some discrepancy between the results obtained in the present study and other previously published studies. The prevalence of peri-implant mucositis was 74.7 % patients and 55.7 % implants. Likewise, peri-implantitis was found in 34.7 % of patients and 20.7 % of assessed implants. Discrepancies with other studies can be associated with factors such as the definition of diagnostic criteria of pathologies (when not standardized), implant systems used, demographic characteristics of the population, follow-up time, environmental factors, composition of the microbiota, size of the sample, radiographic analysis, clinical evaluation, and even type of edentulism (partial or total).
This study allowed to create associations between peri-implant diseases and the general and specific characteristics of the patient. It was possible to evaluate specific characteristics of the implants and their possible associations with peri-implant diseases.
Associated Risk Factors
The literature includes evidence of a potential association between history of periodontal disease (chronic or aggressive) and peri-implantitis in longitudinal and cross-sectional studies making history of periodontal disease a risk factor/indicator for peri-implantitis (17). Karoussis, et al. (20) placed 45 implants in patients with no history of periodontitis, and 8 in patients after successful completion of periodontal therapy. They observed that the incidence of peri-implantitis at 10 years in the group with no history was 6 %, while in the group with a history of periodontitis it was 29 %. In our study, when analyzing the relationship between periodontal disease and the development of peri-implant disease, patients with a history of periodontal disease showed a high frequency of peri-implant disease (82.4 %). Of the patients with periodontal disease at the time of assessment, 83.3 % had at least one implant with peri-implant disease, showing a higher probability of developing peri-implantitis when suffering from periodontitis.
Although smoking has currently shown a strong association with chronic periodontitis and tooth loss, there is no conclusive evidence that smoking is a risk factor/indicator (17). However, authors such as Lindquist, et al. (21) reported that smokers showed substantially greater crestal bone loss than non-smokers. According to Karoussis, et al. (20), 18 % of implants in smokers develop peri-implantitis, while only 6% of non-smokers develop it. In our study, at the time of evaluation, seven patients (9.33 %) reported being smokers. These patients received 23 implants, of which 91.4 % were diagnosed with peri-implant disease, which gives reasons to associate the habit with the pathology under study. However, the non-association could be related to differences in the categorization of smokers and non-smokers (17).
The existing evidence on the need for a minimum of keratinized mucosa around the implant to maintain peri-implant health is still limited (17). However, different reviews have indicated that with a keratinized mucosa < 2mm there is a greater accumulation of biofilm and inflammation of the peri-implant soft tissue when compared to the presence of a band of keratinized mucosa > 2mm (17). Kungsadalpipob, et al. (22), in a cross-sectional study, reported that sites lacking keratinized mucosa had higher plaque scores and bleeding on probing. However, the differences between probing depth and radiographic bone levels were not statistically significant. Therefore, the absence or reduced width of keratinized mucosa can negatively affect oral hygiene measures, but there is little evidence to establish this as a risk factor for peri-implantitis. In this study, 63.2 % of the implants presented an amount of keratinized mucosa > 2 mm. Of all the implants evaluated, all those showing an amount of keratinized mucosa > 5 mm presented peri-implant disease; among the rest of the implants, a frequency of peri-implantitis was 59.7 % to 88.9 %.
In the literature, the implant diameter has not been assessed as a factor associated with the development of peri-implant diseases. Nevertheless, the relationship of peri-implant disease with respect to the diameter of the implants was a parameter assessed this study showing a growing trend in the frequency of peri-implant disease as the diameter of the implant increased. However, it is not possible to affirm a direct association with peri-implant disease because the bone status at the time of implant placement is unknown.
Regarding the frequency of peri-implant disease according to the type of rehabilitation that received the implants, we found that 3.77 % (12 implants) were overdentures, of which all presented pre-implant disease and no significant differences were observed with the proportion of healthy tissues when characterizing them according to the type of rehabilitation. The association between rehabilitation type and incidence of peri-implant pathologies was the object of a study by Araujo, et al. (23), who analyzed the influence of the characteristics of rehabilitation and peri-implant disease. Those authors found that mechanical complications (fracture, loosening, and maladjustment of the prosthetic components) and characteristics of rehabilitation (full-arch restorations, restorations in acrylic or metal-acrylic materials, implants with machined surface and angled abutments) can lead to an increased risk in the incidence of peri-implant pathology. The high frequency of peri-implantitis in hybrid prostheses in the present study could be explained by the difficulty of performing oral hygiene, which entails the use of these prostheses, and by the acrylic material used in their preparation, which tends to facilitate the accumulation of bacterial plaque due to its own characteristics of roughness and high surface energy. On the other hand, implants rehabilitated through overdentures and implant-supported crowns showed a low frequency of peri-implantitis, which may indicate that easy access to oral hygiene in these types of rehabilitation may favor the stability of peri-implant tissues.
Poor biofilm control, poor oral hygiene, and lack of regular maintenance have a potential association with peri-implantitis. Monk, et al. (24), in a longitudinal study published in 2017, showed the importance of controlling the biofilm to prevent peri-implantitis. The study showed that the incidence of peri-implantitis over a 5-year period was lower in patients who attended maintenance appointments (18 %), when compared to patients who did not attend their appointments (44 %). For this reason, periodic maintenance of patients with implants in the mouth is crucial to prevent the appearance and progression of this pathology (24).
In this study, no appreciable differences were found in the prevalence of peri-implant disease when analyzing variables such as age group and gender. However, there was a tendency for peri-implant disease to occur more frequently in patients of both sexes aged 49 years or younger. In a study on the frequency of peri-implant disease and the analysis of associated risk factors, carried out by Ferreira, et al. (25), no relationship was found between age and prevalence of peri-implant disease. In that same year, Daubert, et al. (26) reported, in a cross-sectional analysis that patients who received implants at younger ages had a higher prevalence of peri-implant disease.
For this reason, in the field of periodontics, it has been convincingly proven that supportive periodontal therapy is essential to prevent the incidence or recurrence of periodontal diseases (27,28). Likewise, the frequency of these therapies must be designed individually for each patient, depending on their risk profile.
There is a problem with the level of evidence and the studies carried out to date in relation to diagnosis, prognosis, and treatment protocols, making the only thing that is clear and certain is that prevention from the planning of implant placement plays a crucial role in the onset and progression of such diseases. It is also important to clarify that the parameters established to determine marginal bone loss and thus achieve the diagnosis of peri-implantitis cannot be considered the same for all implant designs. This is because in implants with platform reduction and a conical connection, it would not be expected to find bone loss associated with the initial process of bone remodeling, but rather, bone gain as reported by some studies (29-32).
CONCLUSION
The total frequency of peri-implant diseases was 81.33% in the patients evaluated. The frequency with respect to the patient variable of peri-implant mucositis and peri-implantitis was 74.7% and 34.7%, respectively. The presence of periodontitis and the absence of supportive maintenance therapy seem to be predisposing factors for the development and progression of the disease. Peri-implant disease does not seem to be unifactorial, since patient-related indicators and clinically associated surgical and prosthetic factors converge, which may contribute to its development, progression and severity.
RECOMMENDATIONS
It is suggested to establish a strict supportive maintenance therapy protocol for patients who have received implants, including motivational processes to increase patient adherence to follow-up and prevent or control symptoms of peri-implant diseases in time before the occurrence of implant loss.
ACKNOWLEDGEMENTS
To the Pontificia Universidad Javeriana for the support and funding of this study. This project received approval from the Research and Ethics Committee of the Pontificia Universidad Javeriana’s Dental School, as stated in minutes No. 014-2015.
References
1. Balshi TJ, Wolfinger GJ, Stein BE, Balshi SF. A long-term retrospective analysis of survival rates of implants in the mandible. Int J Oral Maxillofac Implants. 2015 Nov-Dec; 30(6): 1348-1354. https://doi.org/10.11607/jomi.3910
2. Mayta-Tovalino F, Mendoza-Martiarena Y, Romero-Tapia P, Álvarez-Paucar M, Gálvez-Calla L, Calderón-Sánchez J, Bolaños-Cardenas R, Diaz-Sarabia A. An 11-Year Retrospective Research Study of the Predictive Factors of Peri-Implantitis and Implant Failure: Analytic-Multicentric Study of 1279 Implants in Peru. Int J Dent. 2019 Jun 24; 2019: 3527872. https://doi.org/10.1155/2019/3527872
3. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986 Summer; 1(1): 11-25
4. Smith DE, Zarb GA. Criteria for success of osseointegrated endosseous implants. J Prosthet Dent. 1989 Nov; 62(5): 567-572. https://doi.org/10.1016/0022-3913(89)90081-4
5. Fragkioudakis I, Tseleki G, Doufexi AE, Sakellari D. Current Concepts on the Pathogenesis of Peri-implantitis: A Narrative Review. Eur J Dent. 2021 May; 15(2): 379-387. https://doi.org/10.1055/s-0040-1721903
6. Lindhe J, Meyle J; Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008 Sep; 35(8Suppl): 282-285. https://doi.org/10.1111/j.1600-051X.2008.01283.x
7. Smeets R, Henningsen A, Jung O, Heiland M, Hammächer C, Stein JM. Definition, etiology, prevention and treatment of peri-implantitis--a review. Head Face Med. 2014 Sep 3; 10: 34. https://doi.org/10.1186/1746-160X-10-34
8. Kormas I, Pedercini C, Pedercini A, Raptopoulos M, Alassy H, Wolff LF. Peri-Implant Diseases: Diagnosis, Clinical, Histological, Microbiological Characteristics and Treatment Strategies. A Narrative Review. Antibiotics (Basel). 2020 Nov 22; 9(11): 835. https://doi.org/10.3390/antibiotics9110835
9. Araujo MG, Lindhe J. Peri-implant health. J Periodontol. 2018 Jun; 89 Suppl 1: S249-S256. https://doi.org/10.1002/JPER.16-0424
10. Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, Camargo PM, Chen S, Cochran D, Derks J, Figuero E, Hämmerle CHF, Heitz-Mayfield LJA, Huynh-Ba G, Iacono V, Koo KT, Lambert F, McCauley L, Quirynen M, Renvert S, Salvi GE, Schwarz F, Tarnow D, Tomasi C, Wang HL, Zitzmann N. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018 Jun; 89 Suppl 1: S313-S318. https://doi.org/10.1002/JPER.17-0739
11. Schwarz F, Derks J, Monje A, Wang HL. Peri-implantitis. J Periodontol. 2018 Jun; 89 Suppl 1: S267-S290. https://doi.org/10.1002/JPER.16-0350
12. Linkevicius T, Puisys A, Vindasiute E, Linkeviciene L, Apse P. Does residual cement around implant-supported restorations cause peri-implant disease? A retrospective case analysis. Clin Oral Implants Res. 2013 Nov; 24(11): 1179-1184. https://doi.org/10.1111/j.1600-0501.2012.02570.x
13. Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015 Apr; 42 Suppl 16: S158-71. https://doi.org/10.1111/jcpe.12334
14. Atieh MA, Alsabeeha NH, Faggion CM Jr, Duncan WJ. The frequency of peri-implant diseases: a systematic review and meta-analysis. J Periodontol. 2013 Nov; 84(11): 1586-1598. https://doi.org/10.1902/jop.2012.120592
15. Aguirre-Zorzano LA, Estefanía-Fresco R, Telletxea O, Bravo M. Prevalence of peri-implant inflammatory disease in patients with a history of periodontal disease who receive supportive periodontal therapy. Clin Oral Implants Res. 2015 Nov; 26(11): 1338-1344. https://doi.org/10.1111/clr.12462
16. Derks J, Schaller D, Håkansson J, Wennström JL, Tomasi C, Berglundh T. Effectiveness of Implant Therapy Analyzed in a Swedish Population: Prevalence of Peri-implantitis. J Dent Res. 2016 Jan; 95(1): 43-49. https://doi.org/10.1177/0022034515608832
17. Ogata Y, Nakayama Y, Tatsumi J, Kubota T, Sato S, Nishida T, Takeuchi Y, Onitsuka T, Sakagami R, Nozaki T, Murakami S, Matsubara N, Tanaka M, Yoshino T, Ota J, Nakagawa T, Ishihara Y, Ito T, Saito A, Yamaki K, Matsuzaki E, Hidaka T, Sasaki D, Yaegashi T, Yasuda T, Shibutani T, Noguchi K, Araki H, Ikumi N, Aoyama Y, Kogai H, Nemoto K, Deguchi S, Takiguchi T, Yamamoto M, Inokuchi K, Ito T, Kado T, Furuichi Y, Kanazashi M, Gomi K, Takagi Y, Kubokawa K, Yoshinari N, Hasegawa Y, Hirose T, Sase T, Arita H, Kodama T, Shin K, Izumi Y, Yoshie H. Prevalence and risk factors for peri-implant diseases in Japanese adult dental patients. J Oral Sci. 2017 Mar 31; 59(1): 1-11. https://doi.org/10.2334/josnusd.16-0027
18. Romandini M, Lima C, Pedrinaci I, Araoz A, Soldini MC, Sanz M. Prevalence and risk/protective indicators of peri-implant diseases: A university-representative cross-sectional study. Clin Oral Implants Res. 2021 Jan; 32(1): 112-122. https://doi.org/10.1111/clr.13684
19. Duque AD, Aristizabal AG, Londoño S, Castro L, Alvarez LG. Prevalence of peri-implant disease on platform switching implants: a cross-sectional pilot study. Braz Oral Res. 2016; 30: S1806-83242016000100204. https://doi.org/10.1590/1807-3107BOR-2016.vol30.0005
20. Karoussis IK, Salvi GE, Heitz-Mayfield LJ, Brägger U, Hämmerle CH, Lang NP. Long-term implant prognosis in patients with and without a history of chronic periodontitis: a 10-year prospective cohort study of the ITI Dental Implant System. Clin Oral Implants Res. 2003 Jun; 14(3): 329-339. https://doi.org/10.1034/j.1600-0501.000.00934.x
21. Lindquist LW, Carlsson GE, Jemt T. A prospective 15-year follow-up study of mandibular fixed prostheses supported by osseointegrated implants. Clinical results and marginal bone loss. Clin Oral Implants Res. 1996 Dec; 7(4): 329-336. https://doi.org/10.1034/j.1600-0501.1996.070405.x
22. Kungsadalpipob K, Supanimitkul K, Manopattanasoontorn S, Sophon N, Tangsathian T, Arunyanak SP. The lack of keratinized mucosa is associated with poor peri-implant tissue health: a cross-sectional study. Int J Implant Dent. 2020 Jul 16; 6(1): 28. https://doi.org/10.1186/s40729-020-00227-5
23. de Araújo Nobre MA, Maló P. The influence of rehabilitation characteristics in the incidence of peri-implant pathology: a case-control study. J Prosthodont. 2014 Jan; 23(1): 21-30. https://doi.org/10.1111/jopr.12114
24. Monje A, Wang HL, Nart J. Association of Preventive Maintenance Therapy Compliance and Peri-Implant Diseases: A Cross-Sectional Study. J Periodontol. 2017 Oct; 88(10): 1030-1041. https://doi.org/10.1902/jop.2017.170135
25. Ferreira CF, Buttendorf AR, de Souza JG, Dalago H, Guenther SF, Bianchini MA. Prevalence of Peri-implant Diseases: Analyses of Associated Factors. Eur J Prosthodont Restor Dent. 2015 Dec; 23(4): 199-206.
26. Daubert DM, Weinstein BF, Bordin S, Leroux BG, Flemming TF. Prevalence and predictive factors for peri-implant disease and implant failure: a cross-sectional analysis. J Periodontol. 2015 Mar; 86(3): 337-347. https://doi.org/10.1902/jop.2014.140438
27. Nyman S, Lindhe J, Rosling B. Periodontal surgery in plaque-infected dentitions. J Clin Periodontol. 1977 Nov; 4(4): 240-249. https://doi.org/10.1111/j.1600-051x.1977.tb01896.x
28. Ramfjord SP. Maintenance care for treated periodontitis patients. J Clin Periodontol. 1987 Sep; 14(8): 433-437. https://doi.org/10.1111/j.1600-051x.1987.tb02247.x
29. Salamanca E, Lin JC, Tsai CY, Hsu YS, Huang HM, Teng NC, Wang PD, Feng SW, Chen MS, Chang WJ. Dental Implant Surrounding Marginal Bone Level Evaluation: Platform Switching versus Platform Matching-One-Year Retrospective Study. Biomed Res Int. 2017; 2017: 7191534. https://doi.org/10.1155/2017/7191534
30. Nayak R, Devanna R, Dharamsi AM, Shetty J, Mokashi R, Malhotra S. Crestal Bone Loss around Dental Implants: Platform Switching vs Platform Matching-A Retrospective Study. J Contemp Dent Pract. 2018 May 1; 19(5): 574-578.
31. Schmitt CM, Nogueira-Filho G, Tenenbaum HC, Lai JY, Brito C, Döring H, Nonhoff J. Performance of conical abutment (Morse Taper) connection implants: a systematic review. J Biomed Mater Res A. 2014 Feb; 102(2): 552-574. https://doi.org/10.1002/jbm.a.34709
32. Caricasulo R, Malchiodi L, Ghensi P, Fantozzi G, Cucchi A. The influence of implant-abutment connection to peri-implant bone loss: A systematic review and meta-analysis. Clin Implant Dent Relat Res. 2018 Aug; 20(4): 653-664. https://doi.org/10.1111/cid.12620
Notes
*
Original research.
Author notes
Correspondence: reduenas@javeriana.edu.co; silviarivera@javeriana.edu.co; natalie_rosa@javeriana.edu.co; juan_fernandez@javeriana.edu.co; eduardogazel@gmail.com; monica_p_b@hotmail.com; arodrig@javeriana.edu.co
Additional information
How to cite this article: Dueñas Villamil RE, Rivera Picado S, Rosa Ulloa NG, Fernández Gutiérrez JM, Gazel Gazel E, Pérez Barrantes M, Rodríguez Ciódaro A. Frequency of Peri-implant Diseases and Their Associated Factors. Univ Odontol. 2022; 41. DOI: https://doi.org/10.11144/Javeriana.uo41.fpdf